V. Sethi1, B. Giri1, B. Garg1, M. Tarique1, S. Kurtom1, A. Farrantella1, S. Banerjee1, S. Ramakrishnan1, A. Saluja1, V. Dudeja1 1University Of Miami,Department Of Surgery,Miami, FLORIDA, USA
Introduction: Melanoma, especially when detected at an advanced stage, has a dismal prognosis. Only a few immunotherapeutic agents have demonstrated efficacy against this cancer, often only in a selected group of patients and accompanied with significant adverse effects. Triptolide, a biological derived from Tripterygium wilfordii, and its injectable prodrug, Minnelide have been shown by our group to have remarkable efficacy against pancreatic cancer and is undergoing Phase II clinical trial against advanced gastrointestinal malignancies. We aimed to do a preclinical investigation of triptolide in treating melanoma and to find a mechanistic basis for its action.
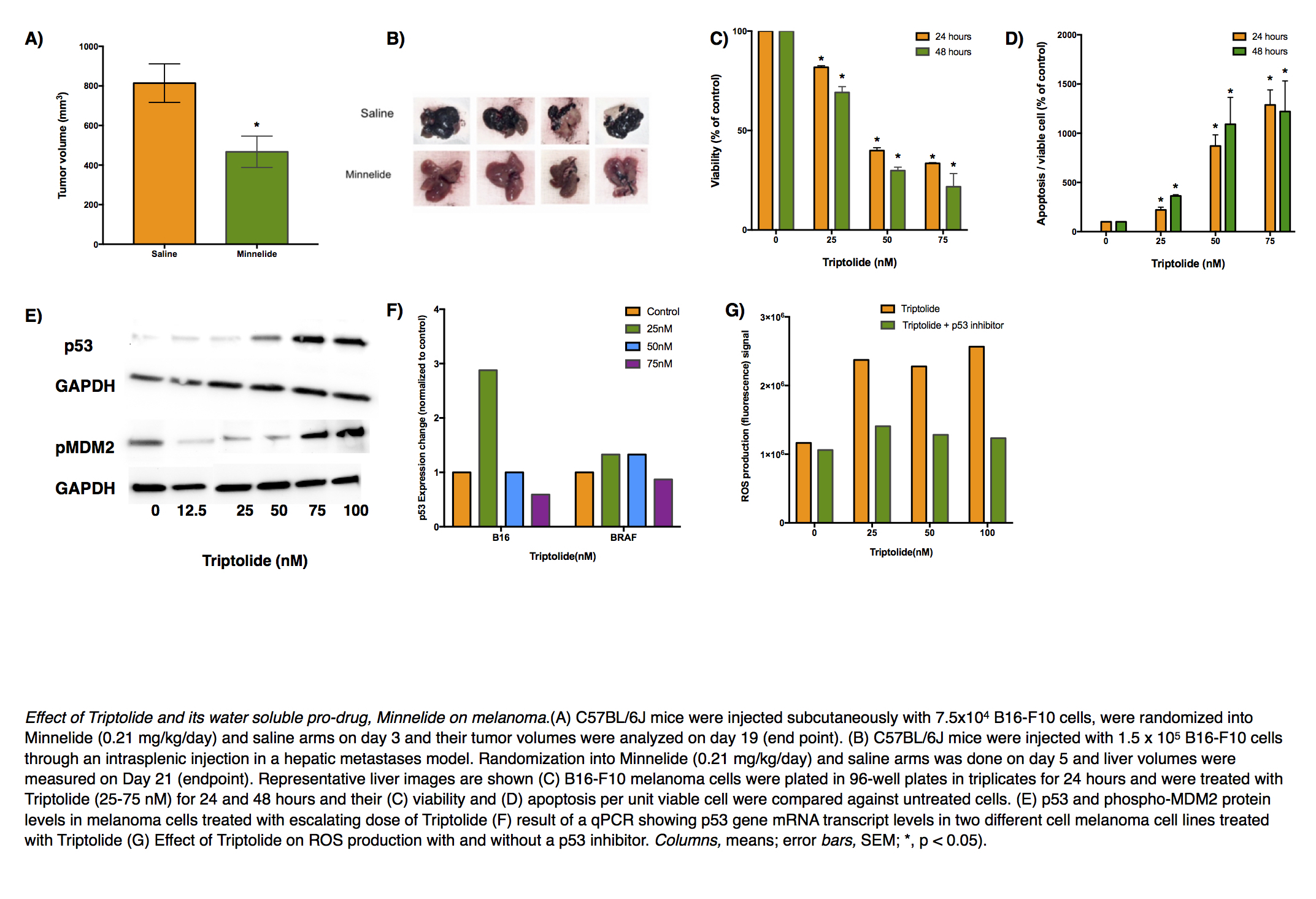
Methods: Melanoma cell lines B16-F10 and a cell line derived from genetically engineered mouse model Tyr::CreER; Braf V600E/+;Ptenlox5/lox5 were used to model skin primary and liver metastases. Minnelide (0.2 mg/kg/day) was given intraperitoneally at a dose that has been shown to be safe in human patients in a Phase I clnical trial. A dose- and time-dependent response of triptolide in determining melanoma cell viability, causing apoptosis, producing reactive oxygen species (ROS) was studied through relevant assays. qPCR and immunoblotting were used to study the effect of triptolide on various genes and proteins. Pifthrin α was used to inhibit the transactivation of p53 responsive genes.
Results: Minnelide treatment caused a significant decrease in melanoma burden in all three preclinical models and resulted in an increase in apoptosis as seen by greater cleaved caspase 3 staining inside tumors. In vitro, triptolide caused a dose- and time-dependent decrease in melanoma cell viability and a simultaneous increase in apoptosis. Triptolide caused ROS generation as early as 1 hour after treatment. It also led to increased levels of γH2AX, suggesting DNA damage, and accumulation of p53. This accumulation was found to be due to decreased ubiquitination of p53 rather than change in the gene expression. Subsequently, inhibition of p53 led to a significant decrease in triptolide-induced apoptosis and ROS generation suggesting a role of p53-dependent apoptosis pathway.
Conclusion: Triptolide and its prodrug, Minnelide, show great promise in forming the basis of an effective chemotherapeutic regimen for treating melanoma.