A. M. Kressel1,3,4, T. Tsaava1, E. H. Chang1, Q. Chang1, V. A. Pavlov1,2, S. S. Chavan1,2, K. J. Tracey1,2 1The Feinstein Institute for Medical Research,Center For Biomedical Science,Manhasset, NEW YORK, USA 2The Feinstein Institute for Medical Research,Center For Bioelectronic Medicine,Manhasset, NEW YORK, USA 3Northwell Health,Department Of Surgery,Manhasset, NEW YORK, USA 4The Elmezzi Graduate School of Molecular Medicine,Manhasset, NEW YORK, USA
Introduction: The inflammatory reflex is a well-defined neural circuit composed of afferent and efferent vagus nerve fibers that both senses peripheral cytokine levels and regulates splenic tumor necrosis factor (TNF) production to maintain homeostasis. Previous studies have demonstrated that efferent vagus nerve signals originate both in the dorsal motor nucleus of the vagus (DMV) and in the nucleus ambiguus in the brainstem. The efferent arc of the inflammatory reflex relays functional signals from the efferent vagus nerve fibers to the splenic nerve, which, after entering the splenic parenchyma, modulates the release of acetylcholine from a subset of T-cells and a subsequent attenuation of TNF production from resident macrophages. Although this pathway has been extensively studied by us and other groups, the existence of a functional synapse between the vagus and splenic nerves remains controversial. Using optogenetics and neural recordings, we selectively studied the role of DMV cholinergic neurons in inducing splenic nerve activation.
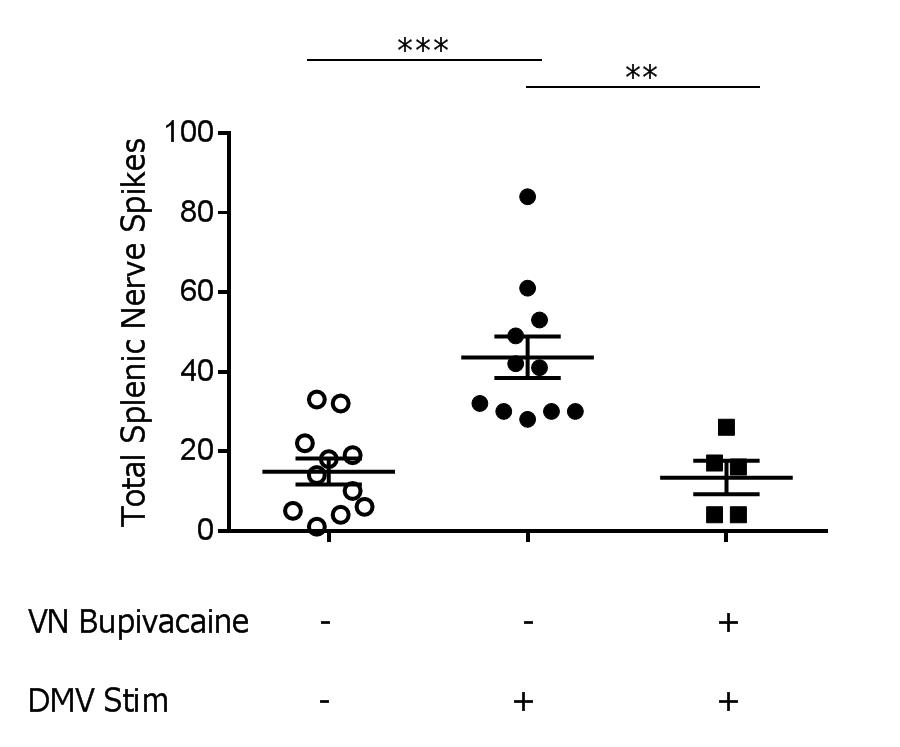
Methods: Using stereotactic guidance, a fiber optic cannula was inserted into the DMV of transgenic mice expressing channelrhodopsin under the choline acetyltransferase promoter (ChAT-ChR2-EYFP) and stimulation was applied (473nm laser, 20Hz, 25% duty cycle, 3 minutes, n=12/group). Splenic nerve activity was simultaneously recorded using a cuffed two-channel electrode. Next, to determine the contribution of the vagus nerve in transmitting signals generated in the DMV to the splenic nerve, we measured splenic nerve activity before and after chemically blocking the vagus nerve. Following recording of splenic nerve activity during DMV stimulation, 0.05% bupivacaine was applied to the vagus nerve to prevent further signal transduction, and splenic nerve activity was recorded again during DMV stimulation.
Results: Splenic nerve activity was significantly increased over baseline (p=0.0002) during optogenetic stimulation of the cholinergic fibers in the DMV (Figure 1). Administration of bupivacaine on the vagus nerve significantly attenuated splenic nerve activity during optogenetic stimulation of cholinergic fibers in the DMV (p=0.0091), demonstrating a functional synapse between the vagus and splenic nerves.
Conclusion: These studies reveal that cholinergic fibers originating in the DMV induce splenic nerve activation via the vagus nerve. Knowledge of the neural circuitry of the inflammatory reflex, coupled with our previous work on endotoxemia, will allow for therapeutic interventions for patients with inflammatory conditions.