K. Strange1, P. Gupta1, A. Carter1, W. Howse1, C. Tan2, H. Ghayee3, K. Pacak4, A. Natarajan5, L. Meijer6, S. Reddy1, J. Bibb1 1University Of Alabama at Birmingham,Department Of Surgery,Birmingham, Alabama, USA 2University Of Texas Southwestern Medical Center,Department Of Psychiatry,Dallas, TX, USA 3University Of Florida & Malcom Randall VA Medical Center,Department Of Medicine, Division Of Endocrinology,Gainesville, FL, USA 4National Institute Of Health,National Institute For Child Health And Human Development,New York, NY, USA 5University Of Nebraska College Of Medicine,Omaha, NE, USA 6ManRos Therapeutics,Roscoff, FRANCE, France
Introduction:
Pheochromocytomas (PCC) are catecholamine-producing tumors arising from chromaffin cells in the adrenal medulla. Approximately 10% of PCC develop metastatic disease having 5-year survival rate <40%. No histopathological criteria exist to predict clinical behavior and current treatments for the malignant form of PCC are ineffective. Cdk5 is a non-traditional CDK family member activated by interaction with non-cyclin co-activators p35/p39. Emerging evidences indicates Cdk5 contributes to the pathophysiology of neuroendocrine (NE) cancers. However, the exact molecular mechanisms by which CDK5 causes tumorigenesis remains elusive. We discovered Cdk5 as a biomarker in different types of NE cancers including PCC. These findings gave rationale to test next generation Cdk5 inhibitors in cells derived from human PCC tumors and explore the mechanisms by which Cdk5 drives PCC cell neoplasia. Moreover, we have begun to model Cdk5-driven PCC in transgenic mice.
Methods:
Well characterized NE cell lines were used including hPheo1, PC12, TT, MTC-SK, SinJ, H1184 and H146. TCGA transcriptome data annotated in UALCAN portal was used to evaluate CDK5 transcript levels. The bitransgenic tetOp system was used to drive p25 expression and induce aberrant Cdk5 activity in mouse NE cells.
Results:
PCC were found to express significantly higher Cdk5 transcript levels compared to its normal counterparts. Protein expression analysis showed similarly elevated Cdk5/p35 in human progenitor PCC cell line (hPheo1) and positive immuno-histochemical staining in human derived tumor tissues. We tested four new analogues that selectively target Cdk5 across panel of human NE cancer cells and assayed the effects on cell viability. All derivatives arrested PCC cell growth with median effective concentrations of 0.02, 2.5, 0.3 and 0.11µM for the respective agents. IC50 values were several-fold lower than the non-selective Cdk5 inhibitor, Roscovitine (26 µM), indicating improved potency. To validate that the growth-inhibitory effects were Cdk5-dependent, we assessed the effect of these agents on novel phosphorylation sites on targets downstream of Cdk5 including pSUV, pH1B, pLARP6 and pRBL1. Quantitative immunoblotting with phosphorylation state-specific antibodies showed attenuation of all phosphosites in hPheo1 cells. Finally, we find that PCC arises in transgenic mice overexpressing aberrant Cdk5 activity, further supporting its role in this disease and suggesting new animal models of PCC will be available to use for the preclinical testing of drugs such as those used here.
Conclusion:
CDK5/p35 is overexpressed in human PCC. Novel Cdk5 inhibitors arrested PPC cell growth and reduced Cdk5-dependent phosphorylation of tumorigenic signaling mechanisms. Thus, these signaling mechanisms that may be critical to PCC initiation/progression.
T. TERAKAWA1,2, E. Katsuta3, M. Fujisawa2, K. Guru1, K. Takabe3 1Roswell Park Cancer Institute,Urology,Buffalo, NY, USA 2Kobe University,Urology,Kobe, , Japan 3Roswell Park Cancer Institute,Surgical Oncology,Buffalo, NY, USA
Introduction:
Solute carrier organic anion (SLCO) genes encode organic anion transport proteins, which is a family of transport proteins that influx number of substrates into the cells including androgens. Among them, high expression of SLCO2B1 has been shown to be associated with the resistance to androgen deprivation therapy and with the prognosis of the patients with hormone sensitive prostate cancer. Here, we hypothesized that the high expression of SLCO2B1 in human prostate cancer may increase influx of androgens to remaining undetectable dormant cancer cells thus is associated with worse disease-free survival after radical prostatectomy.
Methods:
Clinical and RNA-seq data were all obtained from the Cancer Genome Atlas (TCGA). Patients were classified as either high or low expression of SLCO2B1 by the mean value. Overall survival (OS) and disease-free survival (DFS) were compared between high and low expression group in whole cohort, as well as subgroups based upon Gleason score (GS≤6, =7 or ≥8). Gene set enrichment analysis (GSEA) were conducted between high and low expression group.
Results:
Of all patients, 193 and 305 patients were classified as SLCO2B1 high and low expression group, respectively. The patients with high expression of SLCO2B1 were found to have more aggressive cancer characteristics, including high Gleason score (p<0.001), higher T stage (p<0.001), and positive surgical margin (p=0.011). Among all patients, 5-year OS and DFS were 97.8% and 71.2%, respectively. High expression group showed significantly worse DFS after radical prostatectomy (5-year DFS rate: 60.3% vs 78.7%, p=0.026), whereas there was no significant difference in overall survival between these two groups (5-year OS rate: 99.3% vs 96.8%, p=0.923). The patients with higher Gleason score had significantly higher levels of SLCO2B1 expression (GS≤6, vs GS=7; p=0.045, GS=7 vs GS≥8; p=0.002). Significant difference in DFS between high and low expression groups were only observed in the patients with GS≥8 (5-year DFS rate: 38.6% vs 70%, p=0.005), and not in the patients with GS≤7 (GS≤6; p=0.640, GS=7; p=0.923). GSEA demonstrated that in the high expression group of SLCO2B1 enriched KRAS signaling, epithelial mesenchymal transition (EMT) and TGF beta signaling related genes.
Conclusion:
High expression of SLCO2B1 in the prostate cancer patients associated with the aggressive cancer characteristics and recurrence after radical prostatectomy. Furthermore, the higher recurrence rate of the patients with high expression of SLCO2B1 may be able to be explained by its metastatic potential with up-regulated EMT signaling by KRAS and TGF beta pathways.
B. R. Herring2, W. Jason2, S. Jang2, R. Jaskula-Sztul2, H. Chen2 2University Of Alabama at Birmingham,Department Of Surgery,Birmingham, Alabama, USA
Introduction: Ex vivo patient-derived xenografts have the potential to test personalized therapies prior to their administration to the patient. However, techniques to measure cancer cell proliferation in these models are lacking. In this study, we investigated a novel cancer-specific near-infrared (NIR) fluorescent dye, IR-783. Specifically, we hypothesized that IR-783 accurately measures neuroendocrine (NE) cancer cell proliferation in vitro and in vivo.
Methods: NE cancer cell lines (TT, H727, UMCII, MZ, and QGP1) and non-cancerous control cells (HEK293, WI-38 and 917) were plated in culture slides coated with fibronectin. Cells were then incubated with 20 uM IR-783 before fixation and images were acquired with confocal microscopy. Images of single cells were then obtained with an Imagestream Flow Cytometer, and their signal intensities were measured. Uptake of the dye in 2D culture was measured with an In Vivo Imaging System (IVIS) in 12 well plates containing increasing cell number, and the signal intensities were then compared to the results of the MTT assay. Furthermore, NE cancer cells stably transfected with Luciferase were subcutaneously injected into Nu/Nu mice, excised after 7wks of growth, and implanted into a 3D surrogate Bioreactor system for growth up to 20 days. IR-783 was added to the growth medium. The Bioreactor system was exposed to Luciferin before imaging with an IVIS for Luciferase activity and IR-783 uptake.
Results: IR-783 was retained to a higher degree in NE cancer cells compared to non-cancerous cells as detected by confocal microscopy and flow cytometry. NE cancer cells exhibited a mean maximum pixel intensity (mMPI) of 247 while non-cancerous control cells showed an mMPI of 103 (P=.015). In 2D culture, IR-783 signal intensity increased with increasing cancer cell density. This correlation was also shown in the 3D surrogate Bioreactor system (R2=0.49 and 0.96 for IR-783 signal and Luciferase activity, respectively)
Conclusion: As IR-783 is more avidly internalized by NE cancer cells compared to non-cancerous cells, it is a reliable indicator of changes in NE cancer cell number in both 2D culture and the 3D Bioreactor system. It could serve as a powerful tool for detecting the cytotoxic effects of drug candidates in the 3D Bioreactor system for NE cancer cells derived from patients.
S. Ito1, T. Fukagawa2, T. Sato3, T. Masuda1, Y. Kuroda1, H. Eguchi1, M. Sasako4, K. Mimori1 1Kyushu University Beppu Hospital,Department Of Surgery,Beppu, OITA, Japan 2National Cancer Center Hospital,Gastric Surgery Division,Tokyo, , Japan 3Kyushu University,Medical Institute Of Bioregulation,Fukuoka, Fukuoka, Japan 4Hyogo College Of Medicine,Department Of Surgery,Nishinomiya, HYOGO, Japan
Introduction: Liquid biopsy, which is less invasive and simpler method than tissue biopsy, in cancer patients has received enormous attention because of its significant clinical implications. We have reported the relationship between various cancer-related genes expression in preoperative peripheral blood (PB) and bone marrow (BM), and patient prognosis in gastric cancer (GC) patients who underwent surgery. In the current study, we investigated which marker shows the strongest prognostic marker in GC patients.
Methods: mRNA levels of oncogenic genes (BMI-1, JUN, CDC42, PLD1, FOS, FOSB, S1PR1), tumor suppressor genes (FBXW7, MIR-34c), cancer stem cell marker (CD44), circulating tumor cell marker (PLS-3) and immune related genes (PD-1, PD-L1, CD8) in PB and BM samples from 415 GC patients before surgery were investigated by quantitative RT-PCR. For survival analysis, cases were divided into two groups using minimum P value approach based on each gene expression level. Flow cytometric analysis was performed to identify PD-1-expressed cells in peripheral blood mononuclear cells.
Results: Multivariate analysis showed that mRNA levels (high/low) of FOS (HR 0.52, P < 0.05), CD44 (HR 2.57, P < 0.05), PD-1 (HR 0.41, P < 0.001), PD-L1 (HR 1.91, P < 0.01) and CD8 (HR 0.54, P < 0.05) in PB sample, and mRNA levels (high/low) of BMI-1 (HR 0.37, P < 0.05), CDC42 (HR 4.12, P < 0.01), PLD1 (HR 0.50, P < 0.05), FOS (HR 0.51, P < 0.05), S1PR1 (HR 0.23, P < 0.01), FBXW7 (HR 0.49, P < 0.05), CD44 (HR 0.35, P < 0.01), PD-1 (HR 1.75, P < 0.01) and PD-L1 (HR 1.56, P < 0.05) in BM sample were independent factors for overall survival (OS). The strongest independent factor for OS was mRNA levels of PD-1 in PB. PD-1 mRNA levels in PB of GC patients were significantly higher than those of healthy volunteers (n=23); 4.2-fold increase was observed (P < 0.0001). PD-1 mRNA levels in PB of GC patients with neoadjuvant chemotherapy (NAC) (n=15) were significantly lower than those in GC patients without NAC (n=392) (P < 0.01). The proportion of CD3-positive cells (CD4+ and CD8+ T cells) in PD-1-positive cells was 95.4 ± 6.9% in GC patients, suggesting that most PD-1-expressed cells were T cells. Taken together, PD-1 mRNA levels were most associated with survival among cancer- and immune-related genes examined. Since PD-1 is reported to mainly express on activated CD4+ T cells and CD8+ T cells which are closely associated with immune response to tumor, our findings that PD-1 mRNA levels which mostly express on T cells in protein levels in PB were overexpressed in GC patients, and were decreased in patients with NAC, suggest that PD-1 mRNA levels in GC patients may reflect antitumor immune response and immunocompromised condition with NAC.
Conclusions: The strongest prognostic factor was preoperative PD-1 mRNA levels in PB for GC patients who underwent surgery, and may reflect antitumor immune response.
V. Sethi1, B. Giri1, B. Garg1, M. Tarique1, S. Kurtom1, A. Farrantella1, S. Banerjee1, S. Ramakrishnan1, A. Saluja1, V. Dudeja1 1University Of Miami,Department Of Surgery,Miami, FLORIDA, USA
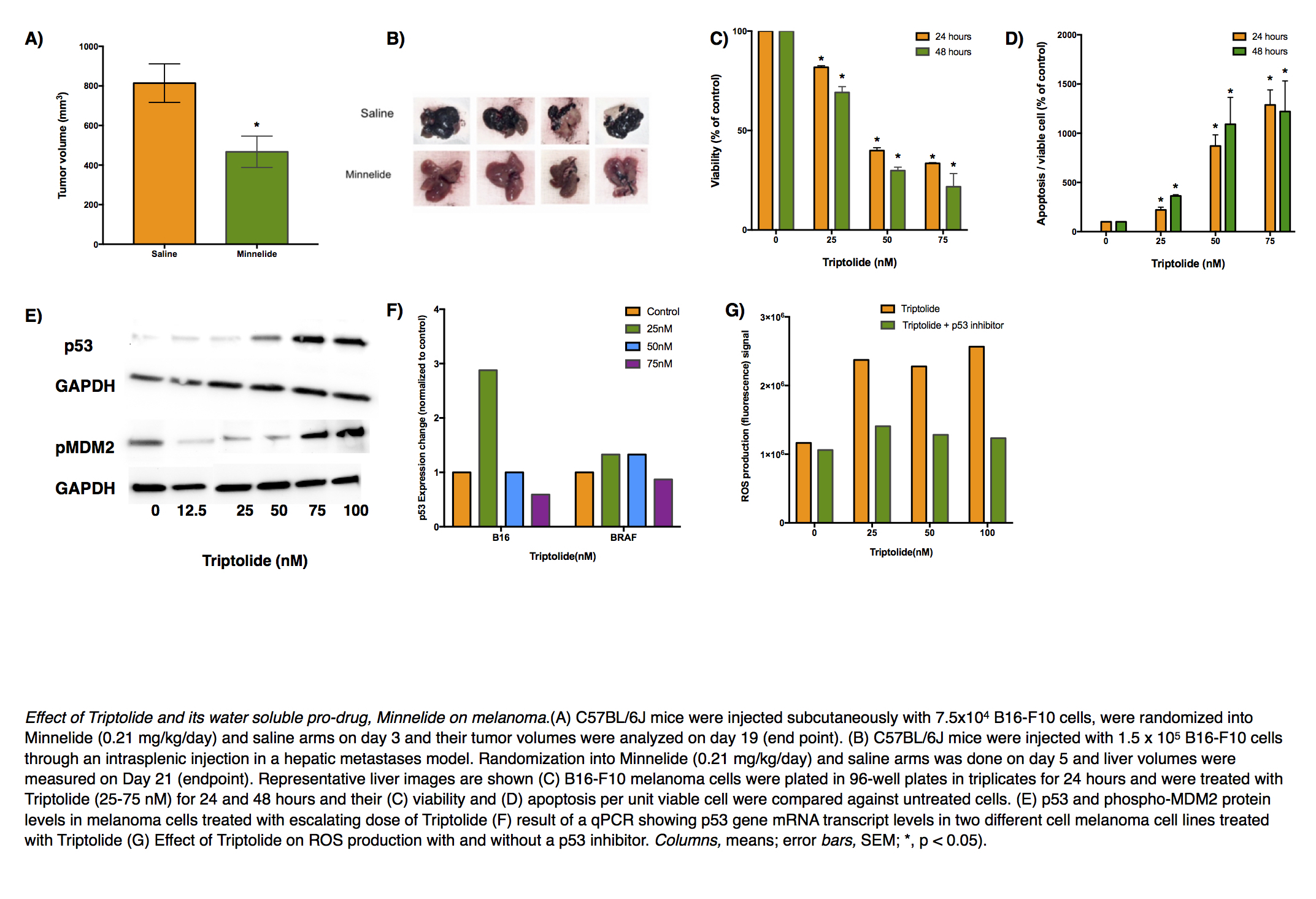
Introduction: Melanoma, especially when detected at an advanced stage, has a dismal prognosis. Only a few immunotherapeutic agents have demonstrated efficacy against this cancer, often only in a selected group of patients and accompanied with significant adverse effects. Triptolide, a biological derived from Tripterygium wilfordii, and its injectable prodrug, Minnelide have been shown by our group to have remarkable efficacy against pancreatic cancer and is undergoing Phase II clinical trial against advanced gastrointestinal malignancies. We aimed to do a preclinical investigation of triptolide in treating melanoma and to find a mechanistic basis for its action.
Methods: Melanoma cell lines B16-F10 and a cell line derived from genetically engineered mouse model Tyr::CreER; Braf V600E/+;Ptenlox5/lox5 were used to model skin primary and liver metastases. Minnelide (0.2 mg/kg/day) was given intraperitoneally at a dose that has been shown to be safe in human patients in a Phase I clnical trial. A dose- and time-dependent response of triptolide in determining melanoma cell viability, causing apoptosis, producing reactive oxygen species (ROS) was studied through relevant assays. qPCR and immunoblotting were used to study the effect of triptolide on various genes and proteins. Pifthrin α was used to inhibit the transactivation of p53 responsive genes.
Results: Minnelide treatment caused a significant decrease in melanoma burden in all three preclinical models and resulted in an increase in apoptosis as seen by greater cleaved caspase 3 staining inside tumors. In vitro, triptolide caused a dose- and time-dependent decrease in melanoma cell viability and a simultaneous increase in apoptosis. Triptolide caused ROS generation as early as 1 hour after treatment. It also led to increased levels of γH2AX, suggesting DNA damage, and accumulation of p53. This accumulation was found to be due to decreased ubiquitination of p53 rather than change in the gene expression. Subsequently, inhibition of p53 led to a significant decrease in triptolide-induced apoptosis and ROS generation suggesting a role of p53-dependent apoptosis pathway.
Conclusion: Triptolide and its prodrug, Minnelide, show great promise in forming the basis of an effective chemotherapeutic regimen for treating melanoma.
W. Lo1, E. Van Beek1, S. Sinha1, A. Ranjan1, G. Chakraborty2, J. Davis1, R. T. Ripley1, J. Hernandez1 1National Cancer Institute,Thoracic And GI Oncology Branch,Bethesda, MD, USA 2Memorial Sloan-Kettering Cancer Center,Department Of Medicine,New York, NY, USA
Introduction: Recent studies have suggested that metastasis is initiated by a subpopulation of cancer stem-like cells or by cancer cells that revert to the stem-like fate upon invasion into the stroma of the target organ. Like normal adult stem cells, the metastasis-initiating cells appear to enter quiescence and undergo reactivation in response to niche signals that are part of the extracellular matrix, embedded in it, or exposed on the surface of adjacent stromal or epithelial cells. The signaling cascades that mediate metastatic reactivation from quiescence remain poorly defined.
Methods: In order to evaluate the signaling mechanisms that differentiate those cells capable of metastatic outgrowth, we designed and implemented an innovative negative selection method. Specifically, we isolated dormant variants of the KPC tumor cells (from Pdx-1-Cre, KrasG12DLSL, Trp53R172HLSL mice) that lack the ability to form liver metastases following splenic injection. We then compared the transcriptomes, secretomes, and exosomes of dormant and fully metastatic cells. Next, we serially examined mouse livers at defined time-points after splenic injection of cells using immunohistochemical and immunofluorescence techniques. Finally, sphere-forming assays were undertaken with and without the addition of extra-cellular matrix proteins. Statistical analysis was undertaken using GraphPad Prism where appropriate.
Results: Comparison of the transcriptomes, secretomes, and exosomes of dormant and fully metastatic cells indicated that the metastatic cells produce and package into exosomes cytokines and matrix proteins able to activate TGF-β signaling. In addition, we have observed that nascent micrometastases coopt the periportal cells and undergo expansion in close apposition with the abluminal surface of blood vessel, which results in vascular occlusion reminiscent of external compression. Intriguingly, the nascent tumors activate local collagen-producing cells before the growing tumor mass egresses and invades/replaces normal hepatic parenchyma. Analysis of an isolated “escape” dormant clone (revertant phenotype), which acquired the capability of forming liver metastasis upon splenic injection, revealed secretion of collagen cross-linking enzymes Loxl-2 and Loxl-3 similar to fully metastatic cells. Accordingly, metastatic cells, but not the dormant cells, were able to augment sphere-forming capacity in the presence of collagen, but no other ECM proteins.
Conclusion: Stromal activation appears to be one of the earliest steps in liver colonization for metastatic pancreatic cancer. Interrupting TGF-β signaling and/or matrix stiffening through collagen cross-linking may be a reasonable strategy for adjuvant therapy after resection of pancreatic cancer.
K. J. Kovatch1, C. Subramanian2, M. E. Prince1, J. Sanchez3, M. S. Cohen2 1University Of Michigan,Department Of Otolaryngology-Head And Neck Surgery,Ann Arbor, MI, USA 2University Of Michigan,Department Of General Surgery,Ann Arbor, MI, USA 3University Of Michigan,Medical School,Ann Arbor, MI, USA
Introduction: Despite advancements in adjuvant treatments for advanced head and neck squamous cell carcinoma (HNSCC), survival has remained relatively unchanged. Current standard of care for HNSCC involves a combination of surgery, chemotherapy, and radiation; however, drug resistance is high and often leads to recurrent or persistent disease. Heat Shock protein 90 (Hsp90) inhibitors have shown promise in the past in HNSCC, but were abandoned due to dose-limiting toxicity (DLT) as a monotherapy. Here, we study a novel, potent Hsp90 inhibitor (that does not elicit the heat shock response and its associated DLT) in combination therapy with current standard of care as a promising new treatment strategy for resistant HNSCC.
Methods: Cisplatin- and cetuximab-resistant cell lines were generated for two HNSCC oral cavity cell lines (UMSCC-103 and UMSCC-108) using co-culture and incremental dosing with each respective drug. Resistance was confirmed by measuring IC50 values using MTS assay. Combination index (CI) was performed with simultaneous treatment of Hsp90 inhibitor (KU757, 0.58-5.0 uM) and either cisplatin (5-20 uM) or cetuximab (3.5-5 uM) for 72 hours. Synergistic effect was defined as CI < 1 using the Chou-Talaylay method. Intrinsic radioresistance of cell lines was characterized using clonogenic assay with treatment dosing of 0-8 Gy.
Results: UMSCC-103 and 108 cell lines were co-cultured with up to 5uM cisplatin and 5uM cetuximab. IC50 values for UMSCC-103 and 108 increased 4- to 6-fold for cisplatin, confirming drug resistance. A modest resistance effect was seen for cetuximab, with 3-fold increase for UMSCC-108 and only marginal increase in UMSCC-103. Combination indices showed synergistic effects for all six cell lines (2 parent, 2 cisplatin-resistant, and 2 cetuximab-resistant) at the doses indicated in Table 1. These results were redemonstrated when cisplatin-resistant lines were treated with KU757 and cisplatin in series, suggesting a sensitizing effect of Hsp90 inhibition. Parent UMSCC-103 and UMSCC-108 cell lines showed radioresistance of 29% and 42% at 2 Gy, respectively. Clonogenic assay with KU757 pre-treatment showed radiosensitizing effect compared to control at 2-8 Gy.
Conclusion: Multidrug therapy inhibits multiple key regulatory pathways simultaneously, thereby lowering drug doses to improve toxicity profiles, and combat development of resistance. These results show for the first time ever synergistic treatment response of Hsp90 inhibition in combination with either cisplatin or cetuximab in both parent and drug-resistant cell lines. Further studies, including functional cellular studies and characterization of altered cellular pathways, are ongoing and may elucidate avenues to overcome mechanisms of resistance.
K. M. Leick1,2, J. M. Obeid2, S. Bekiranov2, C. L. Slingluff2 1University Of Iowa,Iowa City, IA, USA 2University Of Virginia,Charlottesville, VA, USA
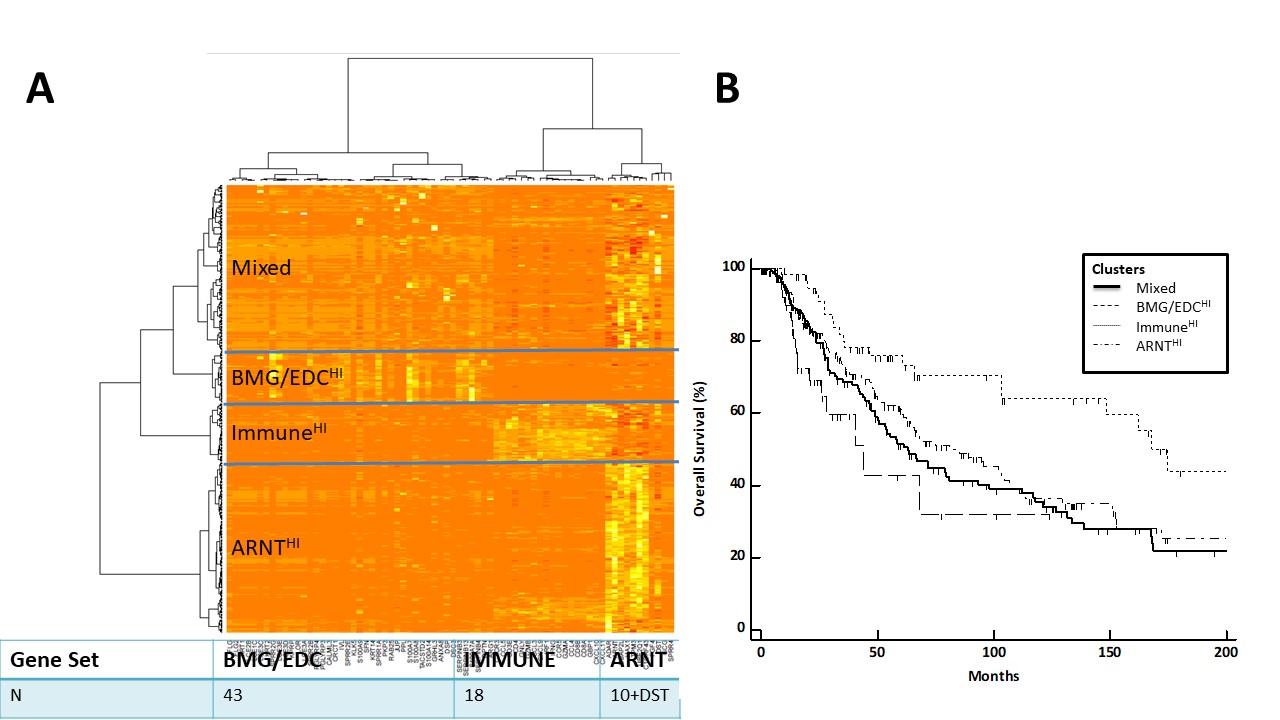
Introduction: We have identified a set of 8 genes expressed in human melanomas and associated with lack of T cell infiltrate and shortened overall survival. These include filaggrin (FLG) and 7 proteins that mediate mechanical barrier function in normal skin through cell-cell adhesions. These barrier molecule genes (BMGs) are concordantly expressed with genes of the epidermal differentiation complex (EDC), which is responsible for terminal differentiation of keratinocytes and is regulated by aryl hydrocarbon receptor (AHR) and AHR nuclear translocator (ARNT). Thus, we hypothesized that AHR/ARNT genes would be expressed concordantly with BMG and EDC genes and would also be negatively associated with immune signatures in melanoma.
Methods: RNA-seq data from 471 patients with cutaneous melanoma were available from the Cancer Genome Atlas (TCGA). Our gene search included the EDC pathway, BMGs, and immune genes. Overexpression of selected genes was identified at z = 1.5, and heatmaps were generated using nonsupervised clustering. Associations of clusters with overall patient survival were assessed using Kaplan Meier curves and log-rank tests.
Results: Gene expression profiles divided tumors into 4 categories: (1) ImmuneHI, (2) BMG/EDCHI, (3) ARNTHI, and (4) Mixed (Figure 1A). ARNT-related genes clustered separately from BMG and EDC genes, which were concordantly expressed. ARNTHI demonstrated upregulation of ARNT-related genes with low immune signature and low BMG/EDC expression. BMG/EDCHI had low ARNT-related and immune gene expression in the presence of upregulated BMGs. Both the ARNTHI and BMG/EDCHI tumors had significantly shorter survival than tumors with high immune signatures (p=0.0003, Figure 1B).
Conclusion: Among patients lacking immune signatures, we have identified 3 different patient subsets: (a) ARNTHI, (b) BMG/EDCHI, and (c) mixed gene expression profiles. Overexpression of BMG/EDC genes are associated with worst survival overall. Despite the fact that AHR and ARNT induce BMG/EDG gene expression in keratinocytes, discordant expression of ARNT genes and BMG/EDC genes in melanomas suggests that BMG/EDC gene expression is controlled in an ARNT-independent manner in melanoma. ARNT HI tumors represent another subset of tumors, in addition to BMG/EDCHI tumors, where immune barriers exist. These findings raise the possibility that barriers to immune infiltrates may differ between EDC/BMG HI and ARNT HI groups. Future studies will define the regulation of EDC and BMG expression and how they may interfere with immune cell infiltration and survival of patients with melanoma and other cancers.
H. Ichikawa1, M. Nagahashi1, M. Nemoto1, T. Hanyu1, T. Ishikawa1, Y. Kano1, Y. Muneoka1, Y. Hirose1, Y. Shimada1, J. Sakata1, T. Kobayashi1, H. Kameyama1, T. Kazuaki2,3, T. Wakai1 1Niigata University Graduate School Of Medical And Dental Sciences,Division Of Digestive And General Surgery,Niigata, NIIGATA, Japan 2Roswell Park Cancer Institute,Breast Surgery, Department Of Surgical Oncology,Buffalo, NY, USA 3University At Buffalo Jacobs School Of Medicine And Biomedical Sciences,The State University Of New York, Department Of Surgery,Buffalo, NY, USA
Introduction: Lymphatic invasion and lymph node metastasis are main mode of spread in esophageal squamous cell carcinoma (ESCC). Sphingosine-1-phosphate (S1P), a pleiotropic bioactive lipid mediator, is one of the important molecule in cancer progression. Sphingosine kinase 1 (SphK1) is activated by phosphorylation and produce S1P. Phospho-SphK1 (pSphK1) were also elucidated to confer to the lymphatic spread of cancer in previous studies. However, the significance of pSphK1 in the progression of ESCC have not been fully investigated to date.
Methods: We retrospectively analyzed the cases of 96 patients who underwent esophagectomy without preoperative therapy for ESCC from 2000 to 2008. Immunohistochemistry of the surgically resected specimens was conducted using the primary polyclonal antibody against pSphK1. Sixty-one of the 96 patients (63.5%) had tumors with high pSphK1 expression (pSphK1-high group), and the others had ones with low pSphK1 expression (pSphK1-low group). We compared clinicopathological factors and overall survival after esophagectomy between the two groups.
Results: Pathological N category according to TNM classification of UICC 7th edition was significantly higher in pSphK1-high group (P < 0.01). Median number of lymph node metastasis (pSphK1-high: 2 vs. pSphK1-low: 0; P < 0.01), the proportion of tumor with lymphatic invasion (67.2% vs. 17.1%; P < 0.01) and that with intramural metastasis (26.2% vs. 2.9%; P < 0.01) were significantly higher in pSphK1-high group than in pSphK1-low group. Multivariate analysis revealed that the presence of lymphatic invasion was independently associated with high expression of pSphK1 (Odds ratio = 8.45; P < 0.01). The 5-year overall survival rate after esophagectomy in the pSphK1-high group was significantly lower compared to the pSphK1-low group (50.8% vs. 67.3%; P = 0.01).
Conclusions: We provide the first evidence of the association between high expression of pSphK1 and lymphatic spread in ESCC. Our study demonstrated that S1P signaling pathway is worth investigating to further understand the molecular mechanism of lymphatic spread in ESCC.
T. Hoki1, T. Yamauchi1, C. A. Eppolito1, A. J. Francois1, K. Odunsi1,2,3, F. Ito1,4,5 1Roswell Park Cancer Institute,Center For Immunotherapy,Buffalo, NY, USA 2Roswell Park Cancer Institute,Department Of Gynecologic Oncology,Buffalo, NY, USA 3Roswell Park Cancer Institute,Department Of Immunology,Buffalo, NY, USA 4State University Of New York At Buffalo,Department Of Surgery, University At Buffalo Jacobs School Of Medicine And Biomedical Sciences,Buffalo, NY, USA 5Roswell Park Cancer Institute,Department Of Surgical Oncology,Buffalo, NY, USA
Introduction:
Cancer neoantigens are derived from nonsynonymous, tumor-specific mutations that create de novo epitopes for T cells, and bypass central thymic tolerance. Although they are highly immunogenic and induce immune responses in humans, the overall success of vaccination studies that target cancer neoantigens has so far been limited. To boost cell-mediated immunity against epithelial tumors, signaling through CD40 has been used with promising results. Toll-like receptor (TLR) agonists have also been implemented as adjuvants. Furthermore, combinatorial stimulation of TLRs and CD40 generates expansion of CD8+ T cells targeting nonmutated self-antigens compared with either agonist alone. However, therapeutic efficacy of combined TLR/CD40 stimulation in the setting of neoantigen vaccine remains elusive.
Methods:
To this end, we used murine MC38 colon adenocarcinoma cells that harbor a single-epitope mutation within the Adpgk protein with the neo-epitope presented in MHC-I H-2Db molecules. C57BL/6 mice were inoculated subcutaneously with MC38 cells. MC38 tumor-bearing mice were treated with soluble Adpgk mutant epitope in combination with TLR agonist, anti-CD40 antibody or both.
Results:
Therapeutic vaccination with the Adpgk mutant peptide combined with TLR agonist and an anti-CD40 antibody (TLR/CD40) significantly slowed tumor growth and improved survival. This was associated with expansion and terminal differentiation of CD8+ T cells in the periphery as well as in the tumor microenvironment. Significantly increased terminally differentiated neoantigen-specific CD8+ T cells in blood and spleen were identified using the tetramer staining assay.
Conclusion:
These studies provide the rational basis for the use of TLR and CD40 agonists together as essential adjuvants to optimize vaccines designed to elicit therapeutic immunity against cancer neoantigens.
M. Nagahashi1, S. Sato2, K. Yuza1, Y. Shimada1, H. Ichikawa1, S. Watanabe3, S. Okuda4, K. Takabe5,6, M. Tsuchida2, T. Wakai1 1Niigata University Graduate School Of Medical And Dental Sciences,Division Of Digestive And General Surgeyr,Niigata City, NIIGATA, Japan 2Niigata University Graduate School Of Medical And Dental Sciences,Division Of Thoracic And Cardiovascular Surgery,Niigata City, NIIGATA, Japan 3Niigata University Graduate School Of Medical And Dental Sciences,Department Of Respiratory Medicine And Infectious Disease,Niigata City, NIIGATA, Japan 4Niigata University Graduate School Of Medical And Dental Sciences,Division Of Bioinformatics,Niigata City, NIIGATA, Japan 5Roswell Park Cancer Institute,Breast Surgery, Department Of Surgical Oncology,Buffalo, NY, USA 6University At Buffalo Jacobs School Of Medicine And Biosciences, The State University Of New York,Department Of Surgery,Buffalo, NY, USA
Introduction:
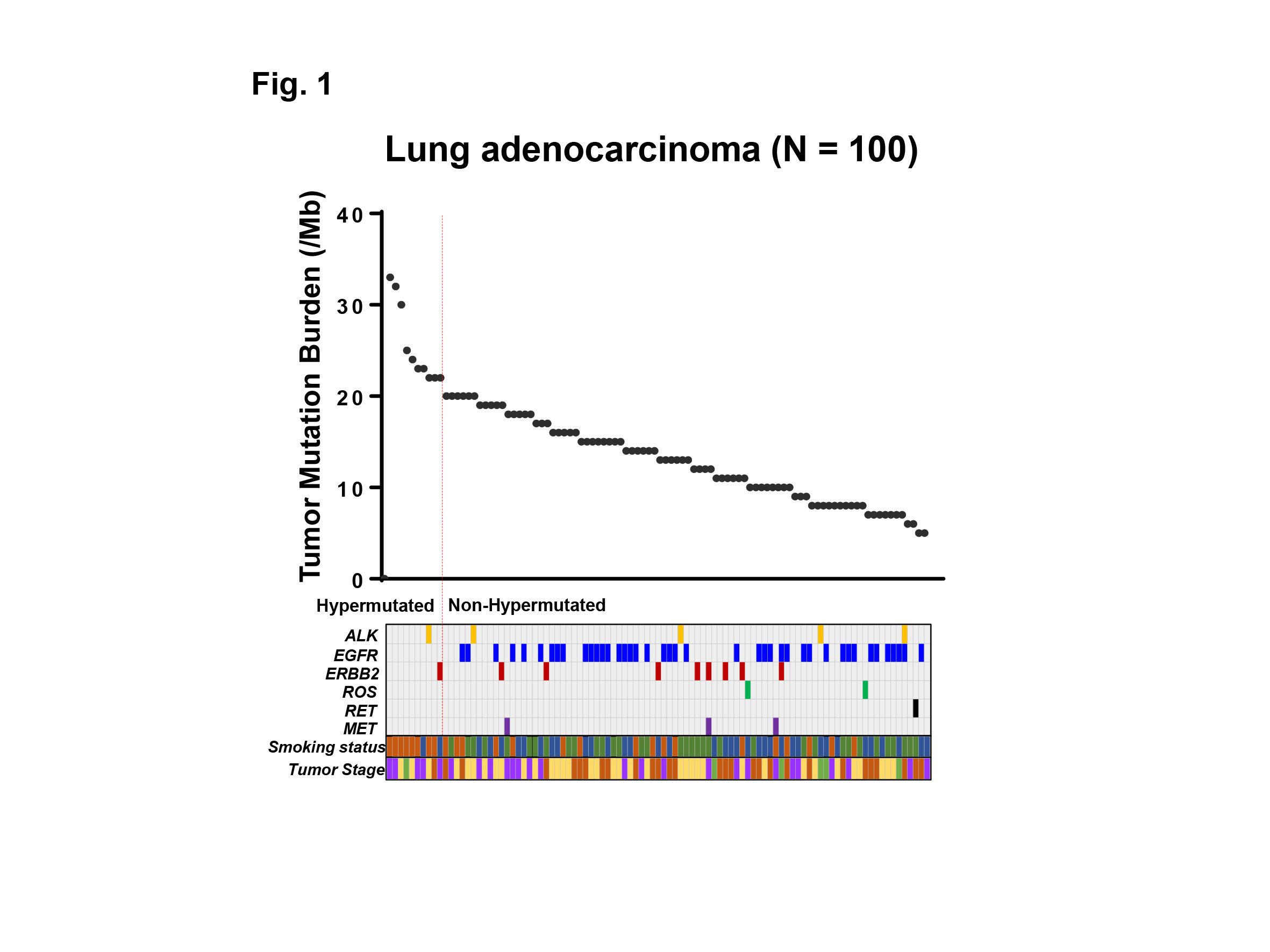
Although the expectation of immune checkpoint inhibitors, such as anti-PD-1 therapy, is tremendously high based on its dramatic efficacy, only a limited number of patients respond. Even worse, there is no definite biomarker to identify which tumor responds to the therapy. Recent progress in the genomic analysis using next-generation sequencing (NGS) technology has enabled comprehensive detection of mutations and tumor mutation burden (TMB) in cancer patients. The high TMB (TMB-H) tumor is defined as one with high somatic mutational rates, which correlates with the generation of neo-antigens and potential clinical response to immunotherapies. In this study, we analyzed the TMB in patients with lung adenocarcinoma utilizing NGS, and clarified the characteristics of patients with TMB-H in relation to common driver mutations of lung adenocarcinoma and smoking history.
Methods:
Genomic aberrations were determined in Japanese patients with lung adenocarcinoma (N=100) using NGS of 415 known cancer genes, and TMB was determined in each patient. High TMB was defined as 20 or more mutations per megabase of sequenced DNA. Common driver mutations, including ALK, EGFR, ERBB2, ROS, RET, MET, and smoking status were compared with TMB to examine their association.
Results:
The median TMB was 13.5 (range 5 – 33) per megabase among 100 patients with lung adenocarcinoma examined. Ten out of 100 (10%) patients showed TMB-H, and 90 (90%) patients showed low TMB (TMB-L) (Fig. 1). Only 2 out of 10 (20%) patients with TMB-H had one of common driver mutations (one had ALK, and the other had ERBB2 mutation), while 57 out of 90 (63%) patients with TMB-L had one of the driver mutations including ALK, EGFR, ERBB2, ROS, RET, MET (P<0.05) (Fig. 1). Of note, no EGFR mutation was observed in patients with TMB-H. Eight out of 10 (80%) patients with TMB-H had current smoking history, while 17 out of 90 (19%) patients with TMB-L had the history, respectively (P<0.001). Moreover, a group of current smokers without driver mutations had the highest TMB in our cohort.
Conclusion:
We analyzed the TMB in patients with lung adenocarcinoma utilizing NGS-based analysis. Our data indicates that the common driver mutations and smoking history are associated with TMB-H in lung adenocarcinoma, which may impact treatment strategies for these patients.
C. J. Corbett1, J. D. Predina3,5,6, A. D. Newton2, M. Shin2, L. Sulyok2, L. Xia2, P. S. Low7,8, S. Singhal1,2,3,4 1University Of Pennsylvania,Perelman School Of Medicine,Philadelphia, PA, USA 2Hospital Of The University Of Pennsylvania,Department Of Surgery,Philadelphia, PA, USA 3Hospital Of The University Of Pennsylvania,Center For Precision Surgery,Philadelphia, PA, USA 4Hospital Of The University Of Pennsylvania,Department Of Thoracic Surgery,Philadelphia, PA, USA 5Harvard School Of Medicine,Brookline, MA, USA 6Massachusetts General Hospital,Department Of Surgery,Boston, MA, USA 7Purdue University,Department Of Chemistry,West Lafayette, IN, USA 8On Target Laboratories,West Lafayette, IN, USA
Introduction:
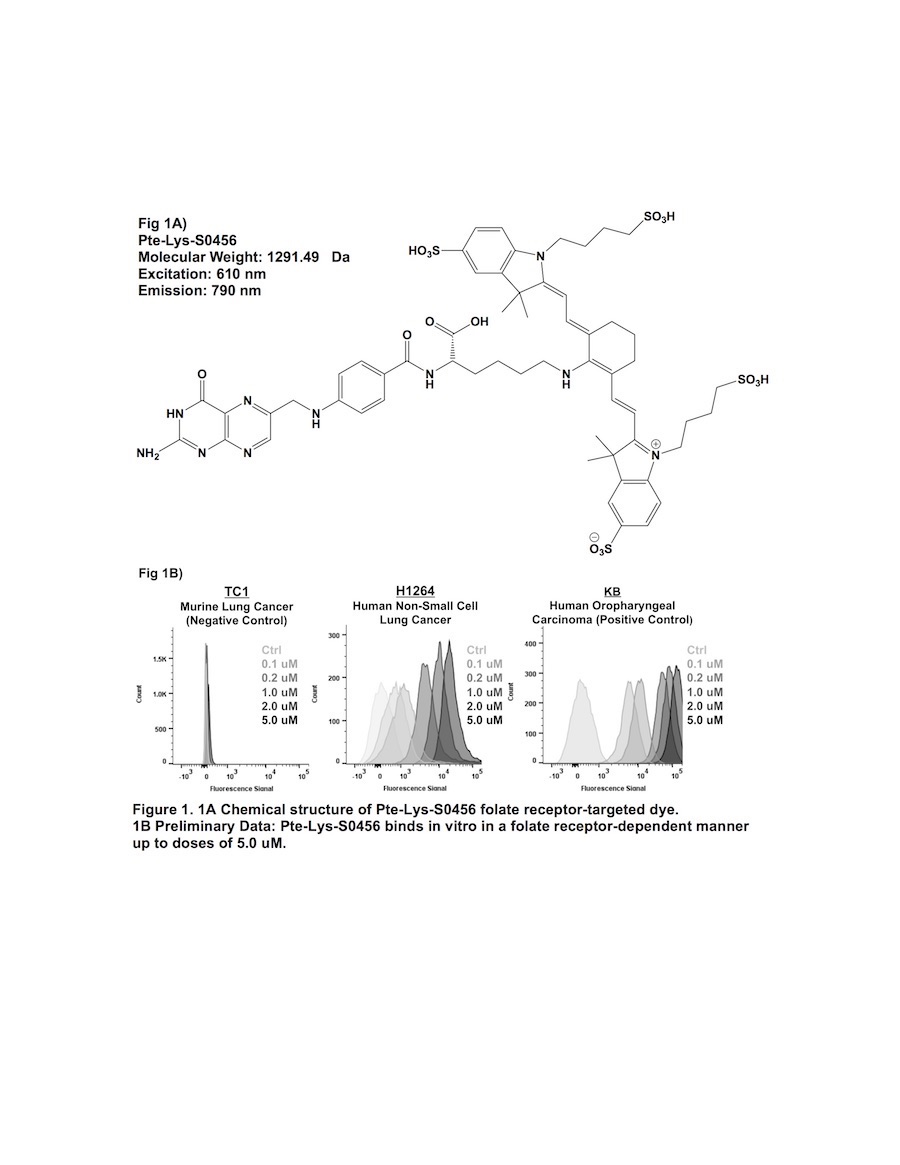
Previous attempts of folate receptor-targeted intraoperative molecular imaging (IMI) of pulmonary adenocarcinomas have been successful, but remain limited by photon-scatter and high levels of tissue reflection. One approach to overcome these limitations is by incorporating fluorophores displaying increased Stokes shifts (greater than 100 nm). In this study, we describe optical properties and preclinical data involving a third-generation folate receptor-targeted probe, Pte-Lys-S0456, that displays a Stokes shift measuring 180 nm.
Methods:
Excitation and emission spectra for Pte-Lys-S0456 were obtained with 10 nM aliquots measured with a luminometer. Next, several murine and human NSCLC models were co-cultured in variable concentrations of Pte-Lys-S0456 ranging from 100 nM to 10 uM for 2 hours. Following washout, fluorescence was assessed with flow cytometry and fluorescence microscopy. Fluorescent patterns were quantified and compared to previous folate receptor-targeted drugs (Folate-FITC and Folate-S0456). Following in vitro characterization, in vivo feasibility of systemc Pte-Lys-S0456 was tested using a small animal flank tumor model of cancer surgery (n=15).
Results:
The peak excitation and emission wavelengths of Pte-Lys-S0456 were found to be 610 nm and 790 nm (Stokes shift of 180 nm). NSCLC models avidly bound Pte-Lys-S0456 in vitro in a folate receptor-dependent manner. Fluorescent signal of tumor cells co-cultured with Pte-Lys-S0456 resulted in increases in fluorescence up to a concentration of 5 uM (p < 0.001). Higher concentrations of Pte-Lys-S0456 did not improve tumor fluorescence. Upon fluorescent microscopic evaluation of tumor models, we observed strong cell membrane binding of Pte-Lys-S0456 in all groups. In cells exposed to the highest concentration, we observed internalization of Pte-Lys-S0456. When compared to Folate-FITC and Folate-S0456, we observed improved signal-to-background signal due to decreased tissue reflection and photon scatter.
Conclusion:
Pte-Lys-S0456 is a novel folate receptor-targeted optical contrast agent that binds NSCLC in a folate receptor-dependent manner. Improved optical properties associated with Pte-Lys-S0456 result in higher in vivo signal-to-background ratios, and thus may provide a more reliable agent for folate receptor-targeted IMI of human NSCLC.
A. A. Gaidarski1, N. Nagathihalli1, M. Van Saun1, N. Merchant1 1University Of Miami,Department Of Surgery,Miami, FL, USA
Introduction: Pancreatic ductal adenocarcinoma (PDAC) is extremely fatal due to its predilection for early metastasis. The hallmark of this tremendous metastatic potential is the epithelial-mesenchymal transition (EMT), signaled by loss of membranous E-cadherin expression. EMT thus represents a crucial target for PDAC therapy but remains elusive due to our poor understanding of its underlying biology. Recently, activation of the Src family of non-receptor tyrosine kinases has been implicated in tumor progression, invasion, and metastasis of PDAC. Moreover, Src has been shown to regulate E-cadherin-induced EMT. The purpose of this study was to examine the effect of Src kinase inhibition on the transcriptional regulation of E-cadherin in PDAC cells.
Methods: Drug-sensitive and -resistant PDAC cell lines were treated with dasatinib (BMS-354825) a novel, multi-targeted kinase inhibitor that targets Src family kinases. The effects of dasatinib on E-cadherin and β-catenin expression were measured by western blot and immunofluorescence for sub-cellular localization. Dasatinib-treated cells were further analyzed for mRNA expression of E-cadherin and its various transcriptional repressors. Finally, E-cadherin promoter activity was measured in both drug-sensitive and drug-resistant PDAC cell lines.
Results: Src kinase inhibition with dasatinib resulted in enhanced E-cadherin and β-catenin expression in the drug-sensitive PDAC cells (BxPC-3 and SW-1990), whereas no change was seen in the more resistant Panc-1 cells. The sensitive cell lines demonstrated an increased expression and membranous localization of E-cadherin and β-catenin upon dasatinib treatment, which was not observed in the resistant cell line. Dasatinib treatment also increased E-cadherin expression at the transcriptional level with a concomitant decrease in the expression of the EMT-associated transcription factor Slug but not LEF-1, ZEB or Snail. Repression of Slug further correlated with an increase in the E-cadherin promoter activity in the sensitive cell lines.
Conclusion: This study elucidates a novel pathway of epithelial-mesenchymal transition in PDAC. In certain populations, Src activation induces the transcription factor Slug, leading to the repression of E-cadherin, precipitating EMT. Dasatinib, a Src-inhibitor, reverses this process and reverts cells back to an epithelial phenotype. Hence, we identify Src as a potential therapeutic target in PDAC and distinguish resistant tumors as those that can upregulate Slug in the face of Src repression.
S. Banerjee1, C. Tang1, M. Yerba1, R. Ustoy1, A. M. Burgoyne2, T. J. Savides3, A. M. Tipps4, J. K. Sicklick1 1University Of California – San Diego,Department Of Surgery,San Diego, CA, USA 2University Of California – San Diego,Department Of Medical Oncology,San Diego, CA, USA 3University Of California – San Diego,Department Of Gastroenterology,San Diego, CA, USA 4University Of California – San Diego,Department Of Pathology,San Diego, CA, USA
Introduction:
Plexiform fibromyxoma (PF) is a rare submucosal gastric tumor that can be confused with gastrointestinal stromal tumor (GIST). While slow growth and lack of metastases suggest an indolent natural history, these so-called benign tumors often present with upper GI bleeding and have a propensity to locally recur. As there are no known drug treatments for PF, resection remains the only treatment. In 2016, the first insight into the molecular biology of 16 PFs demonstrated 4 tumors (25%) with activation of the GLI1 oncogene, a transcription factor in the Hedgehog (Hh) signaling pathway. Despite this discovery, the underlying biology of most PFs remains unknown.
Methods:
Following patient consent to an IRB-approved protocol, clinical data and tumor tissue were collected. After pathologic diagnosis, FoundationOne Heme next generation sequencing (NGS) of >400 genes was performed. Real-time reverse transcription polymerase chain reaction (RT-PCR) for Hh pathway components was performed on mRNA extracted from resected tumor tissue. Additionally, tumor cells were dissociated, placed in primary culture, treated with Hh inhibitor, sonidegib (Novartis/Sun Pharma), and assessed for cell viability using Cell Titer Glo assay.
Results:
We report a 65-year-old male that presented with acute upper GI bleeding from a 5.0 cm gastric mass. Upper endoscopy revealed an ulcerated tumor and biopsy demonstrated a PF. He underwent partial gastrectomy with R0 resection. The immunohistochemical profile (positive: SMA; negative: CD34, S-100, DOG-1, CD117) and histomorphology (transmural myxoid stroma, plexiform growth pattern with spindle cells, and prominent thin capillaries) were consistent with the diagnosis of PF. On NGS, an inactivating Patched 1 (PTCH1) deletion of exons 15-24 was detected. PTCH1, a known tumor suppressor gene implicated in basal cell carcinoma and medulloblastoma tumorigenesis, functions upstream of GLI1 in the Hh pathway. Thus, this tumor’s loss of PTCH1 function can induce downstream GLI1 activation and transcription of target genes. To confirm activation of the Hh pathway in PF, quantitative RT-PCR analysis of mRNA transcripts demonstrated expression of SMO and GLI1, as well as downstream GLI1 transcriptional targets, including Cyclin D1 and HHIP. In turn, treatment with Hh pathway inhibitor, sonidegib (50 μM), demonstrated cell killing with 97.4% efficiency (P=0.0002) after 48-hours.
Conclusion:
For the first time, we report that an inactivating PTCH1 mutation is associated with the development of plexiform fibromyxoma. We show that tumor suppressor gene alterations, rather than oncogenic mutations, within the Hh pathway can also cause plexiform fibromyxoma. In turn, targeted Hh pathway inhibition may represent a viable approach for treating a subset of recurrent plexiform fibromyxomas. Further studies are warranted to investigate the clinical efficacy of these agents in appropriately selected patients.
V. Naga1, C. Gordon1, J. Mazar1, T. Westmoreland1 1Nemours Children’s Hospital,Biomedical Research,Orlando, FL, USA
Introduction: MYCN amplification is a prognostic biomarker associated with poor prognosis of neuroblastoma in children, accounting for 40 to 50 percent of all high-risk cases. The overall survival of children with MYCN amplified neuroblastoma has only marginally improved within the last 20 years. The bromodomain inhibitor JQ1 has been shown to downregulate MYCN in sensitive MYCN amplified neuroblastoma cells (Pussaint et al.). We hypothesize that JQ1 treatment will result in a greater decreased viability in MYCN amplified versus MYCN non-amplified neuroblastoma cells, which, in turn, may have therapeutic implications.
Methods: We used three MYCN amplified neuroblastoma lines [IMR-32, SMS-KAN, and SK-N-Be(1)] and three MYCN non-amplified neuroblastoma lines (SK-N-AS, LAN-6, and CHLA-42) to perform MTS cell viability assays and caspase 3/7 apoptosis assays. Cells were treated at four different concentrations (1 µM, 2 µM, 4 µM, and 8 µM) of JQ1 and its ortho-isomer. Cell viability assays were performed by adding MTS reagent 72 hours after JQ1 treatment and measuring the resulting change in absorbance. For the apoptosis assays, cells were treated at 8 µM of JQ1 and its ortho-isomer. After 48 hours of treatment, a caspase 3/7 substrate was added to the cells and changes in luminescence were measured.
Results: After treatment with JQ1, cell viability decreased in a dose-dependent manner in all cell lines tested. The difference in cell viability between the MYCN amplified and MYCN non-amplified cell lines was most pronounced at the 8 µM concentration of JQ1; cell survival was significantly lower in all the MYCN amplified cells compared to MYCN non-amplified cells. However, responses to the ortho-isomer of JQ1 were not consistent with respect to cell viability. An analysis of the changes in caspase 3/7-dependant apoptosis indicated significant increases in both MYCN amplified and MYCN non-amplified cell lines, though MYCN amplified cells appeared to be more sensitive.
Conclusion: MYCN amplified cell lines demonstrated a more pronounced decrease in cell viability after treatment with JQ1 than the MYCN non-amplified cell lines. This is supported by the caspase 3/7-dependant apoptosis data, which showed that MYCN amplified cell lines showed higher rates of apoptosis after JQ1 treatment. The ortho-isomer of JQ1 had a limited effect on the induction of apoptosis but had a variable effect on cell viability, which suggests that these changes in cell viability may induce a form of cell cycle arrest, indicating it is not entirely harmless as previously reported. Regardless, these data support the hypothesis that JQ1 may serve as an effective therapeutic in the treatment of MYCN amplified neuroblastoma by decreasing both cell viability and increasing the rate of cellular apoptosis in these high-risk pediatric cancers.
H. A. Frohman1,2, P. G. Rychahou1,2, D. S. Watt3,4, Y. Y. Zaytseva2,5, C. Liu2,3, N. Roller2, K. Wang1, B. M. Evers1,2 1University Of Kentucky,Department Of Surgery,Lexington, KY, USA 2University Of Kentucky,Markey Cancer Center,Lexington, KY, USA 3University Of Kentucky,Department Of Molecular And Cellular Biochemistry,Lexington, KY, USA 4University Of Kentucky,Center For Molecular Medicine, Organic Synthesis Core,Lexington, KY, USA 5University Of Kentucky,Department Of Toxicology And Cancer Biology,Lexington, KY, USA
Introduction: Colorectal cancer (CRC) is the second leading cause of cancer deaths in the US with the majority of deaths due to metastatic disease. Current chemotherapeutic regimens involve highly toxic agents, which limits their utility; therefore, more effective and less toxic agents are required to see a reduction in CRC morbidity and mortality. Novel fluorinated N,N’-diarylureas (FND) were developed and characterized by our group as potent activators of adenosine monophosphate-activated kinase (AMPK) that inhibit cell cycle progression. The purpose of this study was to determine the effect of a lead FND compound, FND4b, either alone or combined with PI103 (a PI3K inhibitor) or SN38 (active metabolite of irinotecan) on cell cycle arrest and apoptosis of established CRC cell lines and primary CRC lines established from patient-derived xenografts (PDXs).
Methods: We tested the effects of FND4b, PI103 and SN38 on cell cycle arrest and apoptosis using commercially available CRC cell lines (HT29 and HCT116) and primary cell lines from PDXs established from patients following surgical resection. Briefly, CRC tissues were implanted into NOD scid gamma (NSG) and PDXs (Pt93, Pt130, Pt2377-Primary Tumor [PT], Pt2377-Liver Metastasis [LM]) were established after sequential generations in NSG mice. These PDX models were authenticated as unique human cell lines and genetic profile of 198 oncogenes was determined by Next Generation Sequencing.
Results: Treatment with FND4b for 24h resulted in a marked induction of phosphorylated AMPK expression and a concomitant reduction in Cyclin D1 expression in all six CRC cell lines. Cleaved PARP expression was also notably increased in CRC cells treated with FND4b, which indicated increased apoptosis. Three of the six CRC cell lines had PI3K mutations (HT29, Pt2377-PT, Pt2377-LM), as determined by oncogenic mutation profiling. Other mutations included KRAS, APC, and BRAF. Regardless of the genetic profile of the CRC cells, FND4b treatment resulted in decreased cell proliferation. When CRC cells were treated with combinations of FND4b, PI103, and SN38, there was no change in cell cycle arrest or apoptosis, as compared to treatment with FND4b alone.
Conclusion: Our findings identify FND4b as a novel and effective inhibitor of CRC growth either alone or in combination with PI103 and SN38. Moreover, FND4b activates AMPK at micromolar concentrations, which consistently result in cell cycle arrest and apoptosis in commercially available and PDX-derived CRC cells. Future studies will delineate the effectiveness of FND4b in an in vivo CRC model.
K. Miura1, M. Nagahashi1, M. Nakajima1, K. Yuza1, J. Tsuchida1, Y. Hirose1, M. Abe2, K. Sakimura2, T. Wakai1 1Niigata University Graduate School Of Medical And Dental Sciences,Division Of Digestive And General Surgery,Niigata, NIIGATA, Japan 2Brain Research Institute, Niigata University,Department Of Cellular Neurobiology,Niigata, NIIGATA, Japan
Introduction: Sphingosine-1-phosphate (S1P) is a pleiotropic lipid mediator that regulates cell survival, migration, angiogenesis and lymphangiogenesis, which are all factors involved in cancer progression. S1P is generated inside the cell by two sphingosine kinases (SphK1 and SphK2) and then exported into the tumor microenvironment. We have reported that SphK1 plays an important role in S1P secretion (J Biol Chem 2010) and cancer progression (Cancer Res 2012, J Surg Res 2016), and that SphK2 has a unique role in regulating cellular functions in the liver (Hepatology 2015). Although S1P can be secreted from both cancer cells and non-cancer components such as immune cells and vascular/lymphatic endothelial cells in the tumor microenvironment (Tumor Biology 2017), the roles of S1P produced by tumor and its microenvironment on cancer progression has not been fully investigated. The aim of this study is to investigate the role of SphKs in tumor and its microenvironment using SphK-knockout (KO) cells and SphK KO mice generated by CRISPR/Cas9 technology.
Methods: We generated E0771 murine breast cancer cell lines with a CRISPR/Cas9 mediated targeted deletion of the SphK1 or SphK2 gene. To investigate the role of SphK1 or SphK2 in cellular proliferation, we assessed cell growth by a spectrophotometric technique using the water-soluble tetrazolium salt, WST-8. Cell migration was measured by an in vitro scratch assay. In the animal experiments, the SphK1 KO or SphK2 KO E0771 cells were injected into the subcutaneous tissue of chest of C57BL6 mice, and prognosis of C57BL/6 mice were determined.
Results: Utilizing in vitro proliferation assay of WST-8, we observed significantly less proliferation in SphK1 KO E0771 cells and more proliferation in SphK2 KO E0771 cells compared with their corresponding wild-type (WT) cells, respectively. On the other hand, there were no difference of migration between SphK1 KO and the WT or between Sph2 KO and the WT. The animal experiments showed that mice injected with SphK1 KO cells showed smaller tumor with longer survival than those injected with SphK1 WT cells. Mice injected with SphK2 KO cells also showed similar trend with smaller tumor and longer survival than those injected with SphK2 WT cells. We next implanted WT cells into SphK1 and SphK2 KO mice, and less tumors were developed in the SphK1 KO and SphK2 KO compared with the WT mice, respectively. Finally, we implanted SphK1 KO cells into SphK1 KO mice and WT mice, and found that there were much less growth of tumor of SphK1 KO cells implanted in the SphK1 KO mouse. This indicates that S1P is necessary to the tumor growth, which can be provided from cancer and tumor microenvironment.
Conclusion: Our findings indicate that S1P produced by SphKs in tumor and its microenvironment. Targeting both SphKs and S1P signaling pathways not only cancer, but also in tumor microenvironment will be a key to success to develop new targeted therapy to block S1P signaling.
M. A. Alvarez1, S. M. Husain1, L. F. Reed1, P. V. Dickson1,2, J. L. Deneve1,2, D. Shibata1,2, E. S. Glazer1,2 1University Of Tennessee Health Science Center,Memphis, TN, USA 2UT West Cancer Center,Memphis, TN, USA
Introduction: In pancreatic adenocarcinoma (PDAC), TGF-ß is a tumor suppressor known to drive inflammation in the tumor microenvironment (TME). The role of tumor associated macrophages and interleukins in the PDAC TME is not well described though both likely play critical roles. We hypothesized that pre-treatment of a primary PDAC cell line, Panc-1, with combinations of TGF-ß, macrophages, or IL23, would result in variations in tumorigenesis and metastases in an orthotopic murine model.
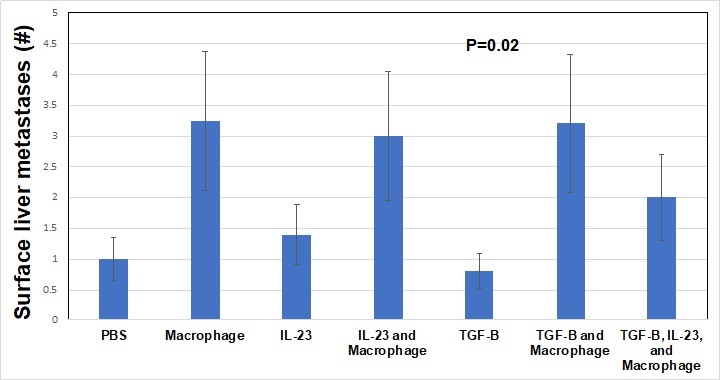
Methods: Panc-1 cells, a line derived from primary pancreatic cancer, were pre-treated with TGF-ß alone (10 ng/mL), IL23 alone (10 ng/mL), macrophages (ATCC cell line, 10:1 ratio of Panc-1 cells to macrophages), IL23 + macrophages, TGF-ß + macrophages, or TGF-ß + macrophages + IL23. Control cells were treated with PBS. Cells were treated twice weekly for 1 week in complete media then implanted into the pancreas of NOD scid gamma mice (NSG, severely immunocompromised) with 5 mice per group. Mouse weights were taken twice weekly for 4 weeks. At that point, mice were sacrificed and tumors harvested. We investigated mouse weight, pancreas tumor weight, pancreas tumor diameter, and the number of surface hepatic metastases. Mean values were compared with ANOVA.
Results: Throughout the study, the mean weight of the mice in the TGF-ß alone and IL23 alone treatment groups was stable whereas all other mice increased weight by 10% (P<0.001). Pancreatic tumor weights were highest in macrophage treatment groups. TGF-ß + macrophage treatment decreased the tumor weight compared to macrophage treatment alone (P<0.001). Macrophage treatment of Panc-1 cells was associated with the highest number of surface liver metastasis after implant (>3 per mouse) and TGF-ß treatment alone was associated with the least number of surface liver metastasis (<1 per mouse, P<0.01). Importantly, TGF-ß treatment did not modulate the rate of macrophage induced surface liver metastasis, but IL23 + macrophage + TGF-ß treatment did result in fewer surface liver metastasis compared to macrophage treatment alone (> 3 vs 2 per mouse, overall P=0.02). Macrophage treated Panc-1 cells were associated with the largest primary Panc-1 tumor growth after 4 weeks, TGF-ß treatment diminished the effect the macrophages had on the Panc-1 cell primary tumor growth (P<0.001).
Conclusion: We found that macrophages drive the development of metastases in this model of early PDAC. While other research groups have shown that TGF-ß is associated with survival in PDAC, we demonstrated that macrophages play a critical role in early PDAC and likely modulate the TME through interleukins such as IL23 and TGF-ß.
E. L. Simmerman1, X. Qin2, J. C. Yu1, B. Baban2 1Augusta University Medical Center,Department Of Surgery / Division Of Plastic Surgery,Augusta, GA, USA 2Augusta University Medical Center,Department Of Oral Biology / Dental College Of Georgia,Augusta, GA, USA
Introduction:
Malignant Melanoma is a complex malignancy with significant morbidity and mortality. The incidence continues to rise and despite advances in treatment, the prognosis is poor. Thus, it is necessary to develop novel strategies to treat this aggressive cancer. Synthetic cannabinoids have been implicated in inhibiting cancer cell proliferation, reducing tumor growth, and reducing metastasis. We developed a unique study focusing on the effects of treatment with a cannabinoid derivative on malignant melanoma tumors in a murine model.
Methods:
Murine B16F10 melanoma tumors were established subcutaneously in C57BL/6 mice. Mice were then treated with intraperitoneal injection of vehicle (PBS) injection (control – group 1, n=6), cisplatin of 5 mg/kg/week (group 2; n=6), and Cannabidiol (CBD) injection of 5 mg/kg twice per week (group 3; n=6) for 14 days. Tumors were measured and volume calculated as 4π/3) x (width/2)2 x (length/2). Tumor size and survival curves were measured. Results were compared using a one-way ANOVA with Multiple Comparison Test.
Results:
A significant decrease was detected in tumor size of mice treated with CBD when compared to the control group (p=0.01). The survival curve of melanoma tumors treated with CBD increased when compared to the control group and was statistically significant (p=0.04). The growth curve and survival curve of melanoma tumors treated with cisplatin were significantly decreased and increased respectively when compared with the control. Mice treated with cisplatin demonstrated the longest survival time but the life quality and movement of CBD-treated mice were significantly better.
Conclusions:
We demonstrate a beneficial therapeutic effect of cannabinoids, significantly influencing the course of melanoma in a murine model. Increased survival and less tumorgenicity are novel findings that should guide research to better understand the mechanisms by which cannabinoids could be utilized for treating cancers. Further studies are necessary to evaluate this potentially new and novel treatment of malignant melanoma.
A. H. Siddiqui1, A. A. Javed3, S. Zafar2 1Aga Khan University Medical College,Medical College,Karachi, Sindh, Pakistan 2University Of Maryland,Department Of Surgery,Baltimore, MD, USA 3Johns Hopkins University School Of Medicine,Department Of Surgery,Baltimore, MD, USA
Introduction:
Cancer surgery is an essential component of healthcare. However, its availability is disparate around the world. Resource mobilization and advocacy requires better measurement of the burden of cancer surgery. We aimed to estimate the global need for cancer surgery and identify disparities by country income status.
Methods:
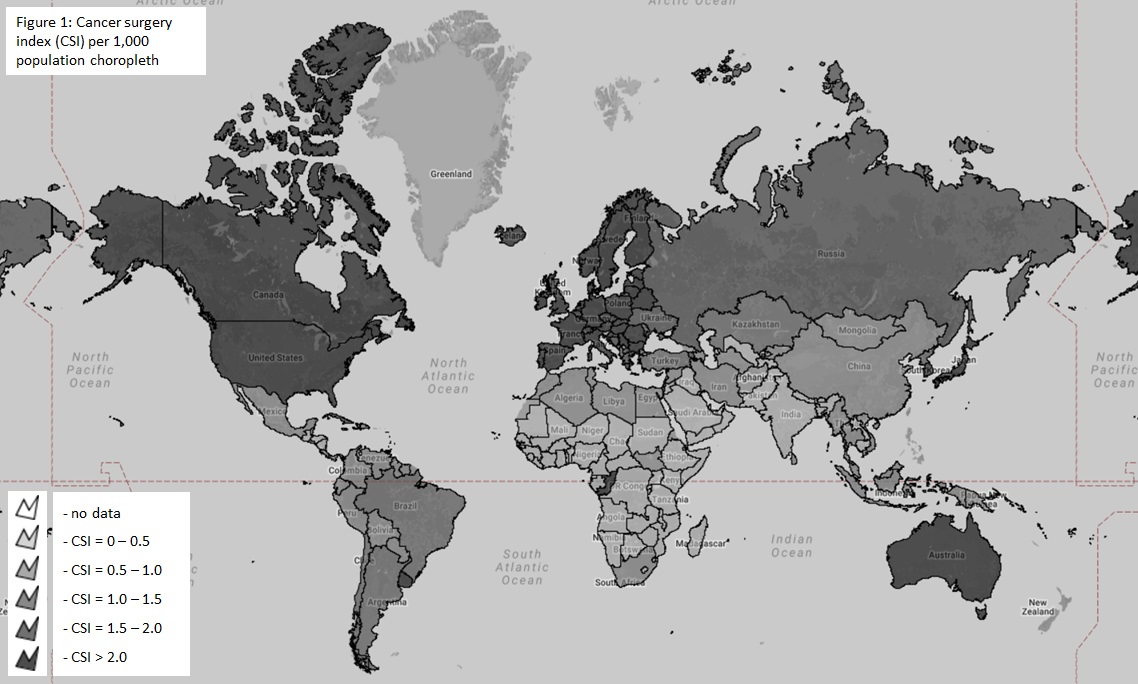
The WHO International Agency for Research on Cancer (IARC) and Global Cancer Observatory (GCO) were queried for data on the incidence of various malignancies in each country. As the incidence of cancer is dependent upon the ability to detect it we only estimate the ‘known’ need for cancer surgery. From the United States Surveillance, Epidemiology and End Result (SEER) database we extracted all patients with a new cancer diagnosis. The proportion of patients requiring surgery for each of these cancers was calculated. This was used to estimate the need for cancer surgery by multiplying with the incidence of each corresponding cancer in each country. The sum for each country was then divided by the population and multiplied by 1,000 to obtain a cancer surgery index (CSI). The Chi square test, t-tests, and Pearson coefficients were used to test associations between CSI and country income, national cancer policy, and presence of cancer registry. Results were tabulated and depicted as choropleth maps using eSpatial (Figure 1).
Results:
The number of people known to be in need of cancer surgery around the world in 2015 was 7,225,695 (± 9,524). The highest need was for breast cancer at 1.17 million patients requiring surgery followed by colorectal cancer (1.06 million). While low and lower-middle income countries make up 48% of the world’s population the reported cancer surgery need was only 21% of the global need highlighting disparities in detecting cancer in resource poor settings. The overall CSI was 0.99 per 1,000 population. The CSI varied almost linearly by income status, with the CSI being 1.95 per 1000 population for high income, 0.85 for upper middle income, 0.53 for lower middle income and 0.29 for low-income countries. Countries with national cancer policies and population based registries had higher CSIs (p<0.01). There was a significant positive relationship between a country’s human development index and the CSI (r=0.7, p<0.01).
Conclusion:
At least 7.2 million people around the world are known to require cancer surgery annually. Variations in the need for cancer surgery are related to a country’s income status, health care expenditure, availability of cancer data, and the presence of cancer control policies. There is an urgent need for systems strengthening in low and middle-income countries to ensure adequate access to cancer surgery.
J. S. Okoroh1,4, S. Essoun3, R. Riviello2, H. Harris1, J. S. Weissman2 1University Of California – San Francisco,Department Of Surgery,San Francisco, CA, USA 2Brigham And Women’s Hospital,Center For Surgery And Public Health,Boston, MA, USA 3University Of Ghana,Korle-Bu Teaching Hospital/ Department Of Surgery,Accra, GREATER ACCRA, Ghana 4Fogarty International Center,UcGloCal Consortium,Bethesda, MD, USA
Introduction:
According to the WHO, essential surgery should be recognized as integral to achieving Universal Health Coverage. We previously reported that surgical conditions were commonly included in national health plans, yet catastrophic health expenditures persist. Insurance is associated with a reduction in maternal mortality and improved access to essential medications in Ghana, but whether it eliminates financial barriers to care for surgical patients is unknown. We sought to describe amounts and payments for general surgical conditions included under Ghana’s national health insurance scheme, and test the hypothesis that insurance protects surgical patients against financial catastrophe.
Methods:
We interviewed patients admitted to the general surgery wards of Korle-Bu Teaching Hospital between February 1 – June 30, 2017 to obtain demographic data, annual income, occupation, household expenditures and insurance status. Surgical diagnoses and procedures, procedural fees, anesthesia fees, medicines and all other costs incurred were collected through chart review. The data was collected on a Qualtrics platform and analyzed in STATA. T-tests and chi-square tests were used to compare insured and uninsured groups. Threshold for financial catastrophe was defined as >10% of annual household expenditures, >40% of non-food expenditures, or >20% of individual income.
Results:
Among 107 enrolled patients, demographic characteristics did not significantly differ between the insured and uninsured except the insured were slightly older [mean 49 years vs 40 years P<0.05.] and more likely to be female [65% vs 40% p<0.05]. The most common surgical procedures for both groups were laparotomy, inguinal hernia repair and appendectomy. Insurance paid on average 40% of the total cost of surgical care, thus protecting some patients from financial catastrophe. However, 50% of the insured patients experienced financially catastrophic payments and almost all reported out-of-pocket payments in addition to hospital payments for medicines and laboratory tests.
Conclusion:
This study—the first to evaluate the impact of insurance on financial risk protection for surgical patients in a resource-limited setting—shows that despite its benefits, about half of insured surgical patients are not protected from financial catastrophe under the Ghanaian national health insurance scheme due to out-of- pocket payments. Government-specific strategies to enroll uninsured individuals at the point of care and to increase the proportion of cost covered are crucial to protecting individuals from financial catastrophe due to surgical care in Ghana thus achieving Universal Health Coverage.

P. F. Johnston1, S. Jalloh2, A. Samura3, J. A. Bailey1, M. Brittany4, Z. C. Sifri1 1Rutgers New Jersey Medical School,Surgery,Newark, NJ, USA 2College Of Medicine And Allied Health Sciences,Freetown, WESTERN, Sierra Leone 3Kabala Government Hospital,Kabala, KOINADUGU, Sierra Leone 4University Of Maryland – Mercy Medical Center,Baltimore, MD, USA
Introduction:
There exists a disproportionally large burden of surgical disease in low income countries (LICs) but few immediate answers. In Sierra Leone, a handful of trained surgeons serve a country of over 6 million, leaving an excess of surgical burden, particularly in rural regions. This excess burden is borne by non-surgeon physicians and surgically-trained clinical officers (COs). In Sub-Saharan Africa, task-sharing models of CO training have shown some success in the context of caesarian sector. However, limited data exists regarding the contribution of surgical training programs towards tackling the general surgery burden of disease. The aim of this study is to examine the impact of one surgically trained CO on surgical capacity in a district hospital in rural Sierra Leone.
Methods:
Kabala Government Hospital (KGH) is a 100-bed district hospital in the rural Koinadugu district of Sierra Leone serving a population of approximately 325,000. The surgical team consists of one non-surgeon physician, one nurse anesthetist, and a handful of COs with various levels of training in surgery and anesthesia. One CO has been trained to perform basic, yet essential, surgery by a non-profit organization operating within Sierra Leone.
Case logs from the KGH operating theater over a 14 month period were reviewed to examine this CO’s contribution to hospital’s surgical output. Two-sided Pearson Chi-square test was performed to determine statistical differences between cases with a physician versus a CO as the primary surgeon.
Results:
In total 394 procedures were performed on 375 patients at KGH over the 14 month period examined. The patient population was primarily male (75%) with a mean age 33.9 ± 18.8. The most common procedures performed were inguinal hernia repair (71%), appendectomy (12%), and hydrocelectomy (9%). Anesthesia was most commonly spinal (50%). The CO was involved in 264 procedures (67%) and primary surgeon for 207 (53%). All cases in the series had a satisfactory immediate surgical outcome as reported in the case logs. No long-term data was available for study.
Physician primaries performed significantly more laparotomies (12% vs. 2%; p = 0.02) than CO primary, but otherwise case types were similar in terms of age, gender, surgery and anesthesia types.
Conclusion:
A surgically-trained CO can significantly enhance the surgical capacity of a district hospital in rural Sierra Leone, performing over half of all operations with satisfactory results. Top down approaches to scaling surgical workforce and infrastructure are costly and will take time, while a large, immediate need exists. Surgical task-sharing programs may be an easily scalable and effective interim solution in areas of excessive burden and limited-resources. Limitations in the complexity of cases performed are expected and likely appropriate.
Long-term and more complete data is needed to ensure quality and safety of surgery performed by graduates of CO training programs.
M. Hamidi1, M. Zeeshan1, N. Kulvatunyou1, T. O’Keeffe1, A. Jain1, A. Tang1, E. Zakaria1, L. Gries1, B. Joseph1 1University Of Arizona,Tucson, AZ, USA
Introduction:
The role of preoperative mechanical bowel (MBP) and oral antibiotic preparation (OAP) in elective colectomy has been studied extensively. However, its role is still unknown in patients undergoing emergent colectomy (EC) for acute diverticulitis. The aim of our study was to determine the association between preoperative MBP and OAP and 30-d outcomes after EC for acute diverticulitis.
Methods:
We analyzed patients from the 2012-15 colectomy-targeted NSQIP database who underwent EC for the indication of acute diverticulitis. Patients were stratified into 1 of the 4 group based on type of preoperative preparation [MBP+OAP, MBP only, OAP only, and no bowel preparation (NBP)]. Multivariate regression analysis was performed to analyze the association between preoperative bowel preparation and 30-d postoperative outcomes. 30-d outcomes were anastomotic leaks requiring intervention, surgical site infections (SSI), hospital length of stay (h-LOS), readmission and mortality.
Results:
3004 patients included. Mean age was 61±14y, and 53% were females. 11% (n=339) patients received preoperative bowel preparation [MBP+OAP (17%), MBP only (38%), and OAP only (45%)]. Most common indication for EC was perforation. Figure 1 demonstrates multivariate regression analysis for 30-d outcomes. Patients who underwent OAP only had lower adjusted rates for anastomotic leaks (OR: 0.7[0.5-0.9]), SSI (0.6 [0.3-0.9]), and readmission (0.6 [0.5-0.7]) compared to NBP. However, patients who received MBP (OR: 1.6 [1.3-2.1]) and MBP+OAP (OR: 1.3 [1.1-1.6]) were more likely to develop postoperative ileus.
Conclusion:
Bowel preparation with oral antibiotics only results in a significantly lower incidence of anastomotic leakage, incisional surgical site infection, and hospital readmission when compared to no bowel preparation. In addition, mechanical bowel preparation might be harmful and reduces the protective effect of oral antibiotic preparation.
J. A. Igu1, C. Haasbroek1, O. C. Nwanna-Nzewunwa1, I. Feldhaus1, M. Carvalho1, M. M. Ajiko2, F. Kirya2, J. Epodoi2, R. Dicker1, C. Juillard1 2Soroti Regional Referral Hospital,Department Of Surgery,Soroti, , Uganda 1University Of California – San Francisco,Center For Global Surgical Studies,San Francisco, CA, USA
Introduction:
Trauma registries (TR) are key components of primary trauma data collection in developing countries. TR implementation can fail if stakeholder involvement is not prioritized. Stakeholder input, is required to create a context-appropriate TR that aptly captures trauma in developing countries. We sought to identify the key components of a context-appropriate prospective TR in a Ugandan Regional Referral Hospital and elicit the determinants of success and sustainability in implementing such a TR.
Methods:
Focus group discussions were held with all cadres of clinicians involved in trauma care delivery at the hospital to identify context-appropriate TR variables. These results informed the design of a TR, which was then implemented. After a one-week pilot of the TR form, we obtained providers’ views on the utility of the TR form by generating a satisfaction score (the average score derived from a five-point Likert scale) for each question.
Results:
Five focus groups consisting of 14 providers (4 intern doctors, 3 Ear-Nose-Throat care providers, 3 general surgeons, 2 orthopedic officers and 2 eye care providers) identified 47 context-appropriate TR variables. Variable categories included: demographics, history and physical exam, injury characteristics, prehospital care, prehospital transportation, investigations, interventions, diagnosis, outcome/discharge status, and consent. These providers listed five barriers to TR implementation: the perception that TRs are time-consuming and increase workload, difficulties following-up admitted patients, lack of personnel, lack of equipment and other resources to gather data, and participation and cooperation issues. They also cited the availability of TR forms distinct from patient forms, TR forms at the point of care, a TR point person, a local TR committee, a good file storage system, and provider TR awareness as facilitators of TR implementation. Providers identified lack of finances, motivation, and salary incentive, and loss of momentum of the TR project as barriers to sustainability. They named the creation and proper training of a local TR team, periodic project evaluation, efficient project resource allocation, creating a research culture, and foreign partnership(s) as facilitators of sustainability. The post-pilot survey captured the perceptions (Figure) of 29 providers (intern doctors, surgeons, clinical officers, nurses) who implemented the TR. Providers were mostly satisfied with the TR form and its implementation.
Conclusion:
Local providers’ perspectives are key to creating context-appropriate and sustainable TRs developing countries, and TR user satisfaction. Having dedicated resources, well-trained local TR staff, and local ownership of the TR is central to TR success.

S. A. Christie1, D. C. Dickson1, T. Nana1, P. M. Stern1, A. Mbiarikai1, R. A. Dicker2, A. Chichom-Mefire3, C. Juillard1 1University Of California – San Francisco,Center For Global Surgical Studies,San Francisco, CA, USA 2University Of California – Los Angeles,Los Angeles, CA, USA 3University Of Buea,Department Of Surgery And Obstetrics- Gynecology, Faculty Of Health Sciences,Buea, SOUTHWEST REGION, Cameroon
Introduction:
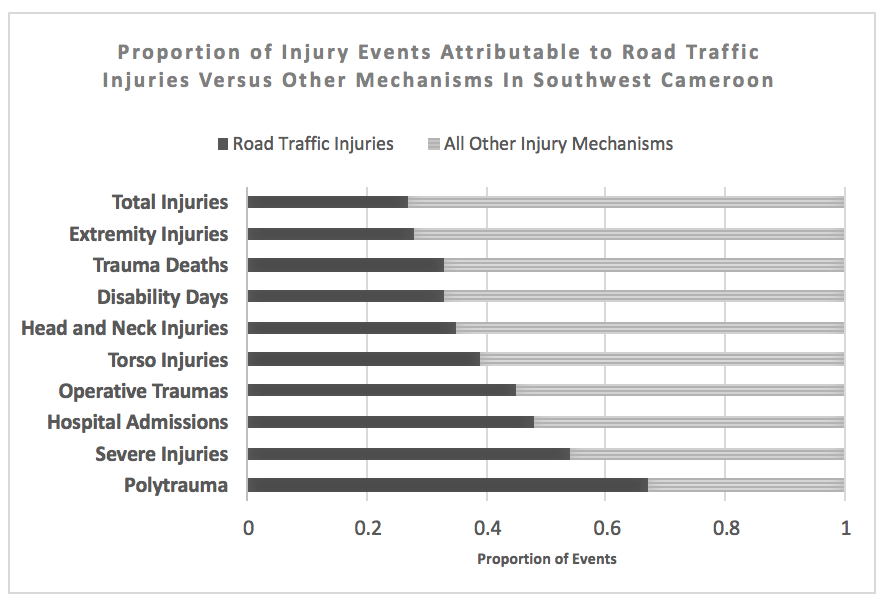
Road traffic injury (RTI) is believed to be a major contributor to death and disability in sub-Saharan Africa. Existing data are predominantly derived from hospital or police records, leading to underreporting in areas where many people do not access formal care. To fill this epidemiologic gap and inform prevention policy, we conducted a community-based survey to identify the yearly incidence, patterns, and impact of road traffic injury in Southwest Cameroon.
Methods:
Three-stage cluster sampling with selection probability proportionate to population was used to select 36 enumeration areas in Southwest Cameroon. Household representatives at each site were asked to report all injuries in the preceding 12 months that resulted in death, loss of routine activity, or required medical attention. Data on injury mechanism, care-seeking behavior, cost of treatment, disability and economic impact were collected.
Results:
Road traffic injury was the largest single-mechanism contributor to trauma-related death and disability. [Figure] Among 8065 individuals in 15 rural and 18 urban areas, 133 RTI were identified for a total incidence of 16.5 RTI /1000 person-years (95CI 14-20). Incidence of fatal RTI was 37/100,000 person-years (95CI 13-105). Although RTI rates were higher in urban areas (18 vs 11/1000 person-years), incidence of RTI death was higher in rural or semirural regions (60 vs 20/100,000 person-years). Commercial transport vehicles were involved in 78% of RTI but few commercial drivers participated in first-aid or victim transport (7.5%). Seatbelts and helmets were very rarely utilized (7.6% and 8.7% respectively). Signs of severe injury including loss of consciousness, confusion, amnesia, or respiratory arrest at the scene occured in 34% of RTI. Formal medical services were sought for 79% of road traffic injuries; among those, 45% were admitted to inpatient care and 8.9% underwent at least one operation. Overall, RTI led to 480 disability days/1000 person-years with 24% of injuries resulting in ongoing disability at the time of the survey. Cost of RTI care was more than double the cost for non-RTI injury mechanisms (64,000 vs. 28,000 CFA, p<0.001) and 46% of RTI resulted in the affected household being unable to afford basic necessities.
Conclusion:
RTI occurs commonly in Southwest Cameroon and results in considerable physical and economic disability. As road safety prevention measures are rarely employed, policy modifications including increased monitoring of seatbelt and helmet compliance and offering first-aid training for commercial vehicle operators represent areas of potential opportunity to reduce disability and injury mortality in Cameroon.