C. Zhang1,2, Y. Li1,2, A. Dawson1,2, P. Ren1,2, L. Zhang1,2, Y. Li1,2, W. Luo1,2, Y. H. Shen1,2,3, S. A. LeMaire1,2,3 1Baylor College Of Medicine,Division Of Cardiothoracic Surgery, Michael E. DeBakey Department Of Surgery,Houston, TX, USA 2Texas Heart Institute,Department Of Cardiovascular Surgery,Houston, TX, USA 3Baylor College Of Medicine,Cardiovascular Research Institute,Houston, TX, USA
Introduction:
Aortic fibroblasts are highly dynamic cell populations that play critical roles in repair and remodeling in aortic aneurysm and dissection (AAD). However, their molecular and cellular changes during AAD development remain poorly understood. In this study, we examined aortic fibroblast heterogeneity in a sporadic AAD mouse model using single-cell RNA sequencing (scRNA-seq).
Methods:
C57BL/6 wild-type (WT) mice were either unchallenged (chow diet, saline infusion; n=3) or challenged (n=3) with 4 weeks of high-fat diet followed by 7 days of angiotensin II infusion. Thoracic aortas were excised and digested to generate single-cell suspensions for scRNA-seq. In all,16,187 cells were analyzed and categorized by using the Seurat package in R to perform cluster identification. Differentially expressed genes (DEGs) were identified using edgeR. Trajectory pseudotime analyses were performed using Monocle2.
Results:
We identified 7 major aortic cell populations including one cluster of Pdgfrahigh fibroblasts. We further divided this cluster into 7 subclusters including extracellular matrix (ECM)-producing fibroblasts (with enrichment in Dcn, Lum, and Col1a2), Acta2+ myofibroblasts, Tnfrsf11bhigh fibroblasts, mesenchymal progenitor cells (with enrichment in Cd34, Ly6a, and Ly6c1), pro-inflammatory fibroblasts (with enrichment in Col8a1, Thbs1, and Tgfb3), and Cd14+ macrophage-like fibroblasts (expressing myeloid-specific markers Lyz2, Cd68, and Pf4). DEGs and Gene Ontology enrichment analyses revealed specific gene expression patterns distinguishing these fibroblast subsets and uncovered their putative functions, such as increased collagen synthesis and ECM organization in ECM-producing and pro-inflammatory fibroblasts, increased cell proliferation and migration features in Tnfrsf11bhigh and pro-inflammatory fibroblasts, and increased cell adhesion and cell junction organization in myofibroblasts and mesenchymal progenitor cells. Notably, pseudotime analyses suggest an important role of Tnfrsf11b in fibroblast trajectory differentiation. Interestingly, while ECM-producing fibroblasts, Acta2+ myofibroblasts, Tnfrsf11bhigh fibroblasts, and mesenchymal progenitor cells were detected in both unchallenged and challenged mice, pro-inflammatory fibroblasts and macrophage-like fibroblasts were almost exclusively detected in challenged mice.
Conclusion:
This study revealed marked phenotypic heterogeneity of aortic fibroblasts and identified changes in fibroblast gene expression during aortic stress, suggesting specialized functions of fibroblast subclusters. Our findings will open up novel opportunities to understand the role of fibroblasts in AAD formation and progression.
K. M. Marsh1, A. Zhang1, D. Wu1, I. L. Kron2, Z. Yang1 1University Of Virginia,Surgery,Charlottesville, VA, USA 2University Of Arizona,Surgery,Tucson, AZ, USA
Introduction: We have identified a novel cardio-splenic axis that is activated and exacerbates myocardial infarct size (IS) during myocardial ischemia-reperfusion injury (IRI). HMGB1 and cell-free DNA (cfDNA) are released from ischemically-injured myocardium into the bloodstream during reperfusion. Facilitated by the interaction between HMGB1 and RAGE on splenic leukocytes, cfDNA enters the leukocytes, stimulates cytosolic TLR9 and augments inflammatory responses, ultimately exacerbating myocardial IS. Hydroxychloroquine (HCQ) is a well-known antimalarial agent and has recently been found to inhibit TLR7/9. We hypothesized that HCQ would attenuate myocardial IRI by inhibiting interferon I-mediated inflammatory responses.
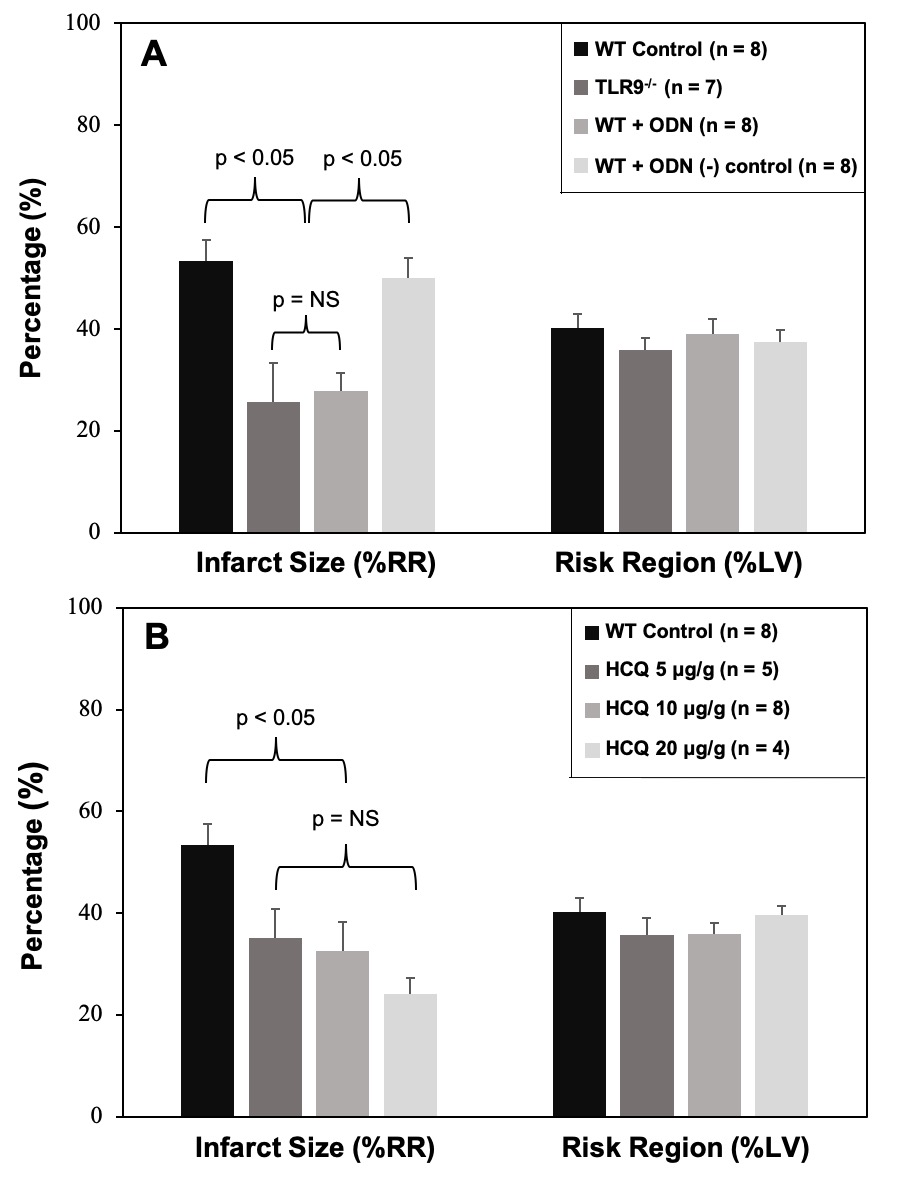
Methods: Wild-type (WT) C57BL/6 and congenic TLR9-/- mice underwent 40 minutes of ischemia following treatment with TLR9 inhibitor ODN-2088, HCQ, or control 5 min prior to left coronary artery (LCA) occlusion. After 60 minutes of reperfusion, hearts were harvested and IS as a percentage of risk region (RR, % of LV) was measured by TTC-Phthalo blue staining. Ex vivo, isolated splenocytes were treated either with PBS or cardiac perfusate (CP) containing high levels of cfDNA and HMGB1 collected from hearts harvested after 40 minutes of LCA occlusion without reperfusion. Cell culture supernatant was then collected at 2 and 4 hours to measure IFNα and IFNβ.
Results: In WT mice treated with ODN-2088, ODN negative control, or PBS control and TLR9-/- mice, there was no significant difference in RR (p=NS). In TLR9-/- mice and OND-2088-treated WT mice (0.6 μ g/g mouse weight), IS was significantly decreased to 26±8 & 28±4% respectively, a >49% reduction compared to WT mice treated with PBS (53±4%, p<0.05, Figure A). ODN-2088 negative control (0.6 μg/g) had no infarct-sparing effect (p=NS, vs. WT control). In WT mice treated with HCQ, RR was comparable among the control and 3 HCQ-treated groups. All 3 HCQ-treated mice (at dosages of 5, 10 and 20 μg/g) had significantly reduced IS (vs. WT control, p<0.05), with the 10 ug/g dose decreasing IS to 33±6% (39% reduction from WT control). A trend towards HCQ dose-response in IS reduction was present but did not reach statistical significance. (Figure B). In the ex vivo experiment, CP-treated splenocytes produced significantly higher IFNα and IFNβ in culture supernatant at 4 hours. HCQ significantly reduced the production of IFNα and IFNβ.
Conclusion: HMGB1 and cfDNA released from ischemic myocardium during reperfusion exacerbate myocardial IS by activating TLR9/IFN I-mediated inflammatory responses. Hydroxychloroquine reduces production of IFN I and attenuates myocardial IRI, likely via TLR9 inhibition.
A. E. Dawson1, Y. Li1, C. Zhang1, P. Ren1, H. Vasquez1, W. Ageedi1, W. Luo1, L. Zhang1, Y. Li1, H. Lu2, L. Cassis3, J. S. Coselli1,4, A. Daugherty2, Y. H. Shen1,4, S. A. LeMaire1,4 1Baylor College Of Medicine,Department Of Surgery, Division Of Cardiothoracic Surgery,Houston, TX, USA 2University Of Kentucky,Saha Cardiovascular Research Center And Department Of Physiology,Lexington, KY, USA 3University Of Kentucky,Department Of Pharmacology And Nutritional Sciences,Lexington, KY, USA 4Texas Heart Institute,Department Of Cardiovascular Surgery,Houston, TX, USA
Introduction:
Marfan syndrome (MFS) is known to be caused by mutations in the gene encoding the glycoprotein fibrillin-1 (FBN1), however the molecular and cellular processes leading to progression of disease remains poorly understood. Aortic endothelial cells (ECs) are vital in the maintenance of the normal aortic wall, with involvement in permeability, cell-cell signaling, and mechanotransduction. Here, we used single-cell RNA (scRNA) sequencing to define the EC populations and cell-specific gene expression in patients with MFS compared to controls, hypothesizing that ECs in MFS would exhibit evidence of endothelial dysfunction.
Methods:
We performed scRNA sequencing of aneurysmal ascending aortic tissues from patients with MFS (n=3) undergoing aneurysm repair and of age-matched non-aneurysmal control tissues from cardiac transplant donors and recipients (n=4). Tissues were digested and single cells isolated and sequenced. In all, over 46,000 cells were grouped into clusters based on similar conserved gene expression using the Seurat package in R. ECs were identified based on high expression of known marker genes, such as VWF, and were re-clustered for dedicated analysis. Differentially expressed genes (DEGs) were identified using edgeR.
Results:
We identified a total of 2,218 ECs in our data which were grouped into 8 clusters. Marker gene expression in each cluster identified EC clusters associated with innate immunity (n=3; marker genes associated with complement pathways and antigen presentation), vascular healing (n=3; marker genes associated with mechanical stress, remodeling, and vascular development), proliferation (n=1; marker genes associated with cell cycle progression), and de-differentiated ECs (n=1; marker genes associated with matrix production and smooth muscle contraction). Module scores of genes involved in tight and adherens junctions were highest in the vascular healing ECs, indicating that these cells were most involved in maintenance of the endothelial barrier. Cells from MFS tissue had a higher proportion of vascular healing ECs compared to controls. Analysis of DEGs revealed that ECs in MFS exhibited significant downregulation of claudin-5 (CLDN5), the predominant tight junction gene expressed in our data. Genes most consistently significantly upregulated in MFS ECs included genes involved in vasculogenesis such as beta-catenin (CTNNB1) and NOTCH1.
Conclusion:
We identified unique subpopulations of aortic ECs using scRNA sequencing. When compared with controls, MFS tissues had a higher proportion of ECs involved in maintaining the endothelial barrier, however had downregulation of CLDN5, the predominant tight junction gene in our data. Additionally, genes involved in vasculogenesis were significantly upregulated. Together, this suggests dysfunction of the endothelial barrier in MFS with increased endothelial cell migration and proliferation.
J. Flaming1, R. Chandra1, L. Girard1, D. Ganguly1, J. Toombs1, J. Minna1, R. Brekken1 1University Of Texas Southwestern Medical Center,Hamon Center For Therapeutic Oncology Research,Dallas, TX, USA
Introduction:
Macrophages are key regulators of the immune landscape within the tumor microenvironment (TME) and the plasticity of macrophage phenotypes in the TME correlates with prognosis in Non-Small Cell Lung Cancer (NSCLC). Depending on their phenotype, macrophages in the TME can secrete pro-tumorigenic cytokines and chemokines, ultimately suppressing the function of other immune cells in the TME. The purpose of our study was to investigate how individual NSCLC cell lines alter macrophage phenotype in tumor cell, cancer-associated fibroblast (CAF) and macrophage co-cultures and to relate effects to the molecular characteristics of different NSCLCs. We hypothesize that immune suppression occurs through tumor-secreted signaling molecules that if identified and blocked, could alleviate macrophage-mediated immune suppression, resulting in improved anti-tumor immune responses
Methods:
We developed an in vitro organoid co-culture system (NSCLC tumor cells, human CAFs, and mouse macrophages) to interrogate cancer cell features causing heterogeneity of macrophage phenotypes across a panel of NSCLCs. We measured (with 4-7 replicates for each NSCLC cell line): mRNA expression in mouse macrophages with a panel of qPCR probes for important macrophage related genes (Arg, NOS2, IL-1β, IL-6, CHIL-3, SOCS3), and in selected cases, whole genome RNAseq; and protein expression using cytokine arrays measuring expression of 40 inflammatory cytokines. Positive controls were stimulation with LPS and IL-4.
Results:
Using our platform, we characterized over 70 NSCLC patient derived lines for their effects on mouse macrophage phenotype. We found: 1) the macrophage phenotypes induced by any one NSCLC were highly reproducible; 2) CAFs are important in the co-culture polarization of macrophages 3) three major clusters of cancer polarized macrophage phenotypes: high Arg (immune suppressive), high IL-1β (inflammatory) or high SOCS3 (JAK-STAT3 pathway) expression; 4) major oncogenotypes (KRAS, TP53, STK11, EGFR, BRAF) do not correlate with the induced macrophage phenotype. We selected 10 NSCLC “exemplars” lines representing each of these 3 clusters for RNA sequencing (mouse genes) and cytokine array protein (human) profiling. Across all clusters we found: 1) suppression of macrophage endocytosis pathways and activation of scavenger receptor A (SRA) signaling (M2 immunosuppressive phenotype); 2) increased expression of human IL6, IL8, and MCP1 proteins, which have been implicated in suppressing innate immune tumor sensing. Analyses of differences between the 3 clusters is ongoing.
Conclusion:
Patient derived NSCLC preclinical models have reproducible effects on macrophage phenotypes in co-cultures. Three major classes of NSCLC cell lines, which are not linked to oncogenotype, initiated reproducible macrophage alteration. Cytokines secreted by NSCLC cell lines appear responsible for these macrophage changes and this system provides an experimental platform to systematically test each as potential therapeutic targets.
N. Haywood1, A. Zhang1, E. Rotar1, S. Noona1, H. Ta1, M. Salmon1, I. L. Kron1, V. E. Laubach1 1University of Virginia Health System,Surgery,Charlottesville, VA, USA
Introduction: Ischemia-reperfusion (IR) injury after lung transplantation is a major cause of early graft dysfunction and increases the risk of early morbidity and mortality. Purinergic P2Y2 receptors (P2Y2R) are G-protein coupled receptors that are known to modulate the inflammatory response by enhancing inflammatory cell infiltration and activation. This study tests the hypothesis that P2Y2R modulates lung IR injury.
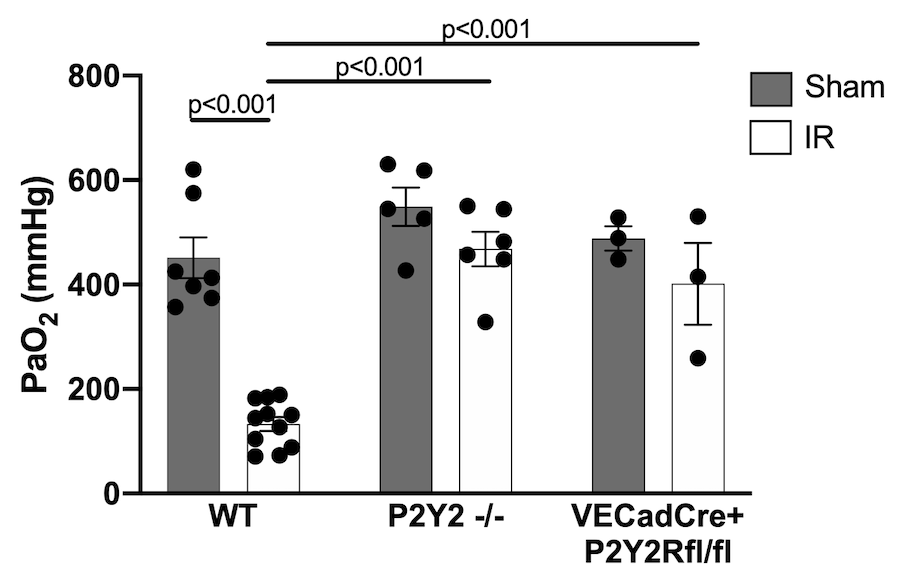
Methods: C57BL/6 wild-type (WT), P2Y2R-/-, and endothelial-specific P2Y2R (VECadCreP2Y2Rfl/fl) mice were subjected to lung IR or sham surgery using an in vivo left lung hilar-ligation model (1-hour ischemia, 2 hours reperfusion; n=3-11 mice/group). Left lung-specific partial pressure of oxygen (PaO2) was then measured to determine pulmonary function. Lung injury was assessed using wet-to-dry weight (WTD) ratio, and lung compliance was measured using a buffer-perfused isolated lung system. Murine lung primary microvascular endothelial cells (PMVEC) were exposed to hypoxia/reoxygenation (HR, 6 hours/4 hours) as a lung IR surrogate and treated with either P2Y2R inhibitor (AR-C 118925XX, 10µM) or vehicle to quantify the secretion of CXCL1 cytokine as an endothelial inflammatory marker. Data is presented as mean +/- SEM.
Results: Compared to WT, significantly improved oxygenation as measured by PaO2 following IR occurred in P2Y2R-/- mice (468.2±33.1 vs 133.1±13.2 mmHg, p<0.001) and in VECadCre+P2Y2Rfl/fl mice (401.3±78.6 vs 133.1±13.2 mmHg, p<0.001) (Figure). Similarly, a significant decrease in lung edema as measured by WTD ratio following IR occurred in P2Y2R-/- mice (4.86±0.15 vs 5.51±0.09, p=0.008) compared to WT. Lung compliance following IR was significantly improved in P2Y2R-/- mice (28.91±0.84 vs 14.86±0.83 µl/cm H2O, p<0.001) compared to WT. In vitro experiments using PMVECs showed a multifold increase in CXCL1 production following HR, which was mitigated with treatment of P2Y2R inhibitor (p=0.025).
Conclusion: Our results suggest that P2Y2R modulates the pulmonary inflammatory response following IR. This modulation is largely due to P2Y2R activity on pulmonary endothelium. P2Y2R may be a promising new therapeutic target to decrease the incidence of primary graft disfunction following lung transplantation.
F. S. Azari1, G. T. Kennedy1, L. Frenzel Sulyok1, M. Bryski1, E. Bernstein1, S. Singhal1 1Hospital Of The University Of Pennsylvania,Thoracic Surgery,Philadelphia, PA, USA
Introduction:
Optimal chance for mesothelioma cure is predicated upon R0 resection and sound surgical principles, which often is not possible due to extensive nature of the disease and inability to discern malignant cells using visual and tactile feedback. Fluorescence guided surgery can ameliorate these challenges and aid in detection of residual disease. The purpose of this study was to evaluate the in-vivo sensitivity of a novel fluorescent dye (VGT-309) in detection of malignant mesothelioma.
Methods:
All experiments were approved by the IRB and were conducted in accordance to policies set forth by the office of University Laboratory Animal Resources. Athymic 15-week old BALB/c female mice were injected with 1 million AB12 cells (mouse derived mesothelioma cells) which were grown in supplemented R10 media. Subsequently, after tumor burden reached 250mm3, the mice were injected with 100 uL of vehicle solution of VGT-309 at differing doses. Animals were then imaged at 15-minutes, 1 hour, 3 hours, 24 hours, and 48 hours using near-infrared (NIR) imaging. The organs and tumor cells were retrieved after 48 hours for histopathological evaluation of dye accumulation. Signal to Background Ratios (SBR) were measured to quantify fluorescence using the ImageJ software (National Institutes of Health).
Results:
All the animal subjects tolerated the procedure well without adverse effects attributable to VGT-309 administration. After 48 hours, tail vein injection at 2mg/kg and 4 mg/kg demonstrated consistent fluorescence in AB12 cells in all the mice under NIR imaging. Strongest fluorescence (15,000 A.U) was detected after 24 hours of drug delivery compared to earlier time points and 48 hours. Furthermore, malignant mesothelial cells showed strongest fluorescence (12,500 absorbance units (A.U)) versus various visceral organs including pancreas, liver, stomach, bowel, and lung with mean A.U of 5300 (Figure 1). Immunohistochemical and histopathological analysis confirmed the selective accumulation of the dye in the tumor compared to background healthy tissue. Mean SBRs for tumor tissues were noted to be 3.48 and 4.12 at 24 and 48 hours respectively.
Conclusion:
We have demonstrated that the novel VGT-309 dye can safely and reliably accumulate in malignant mesothelial cells as well as be detected using conventional fluorescence equipment. Implementation of this dye in surgical treatment of mesothelioma patients can aid in higher chance of R0 resection and subsequently increase the 5-year survival rates. Further studies in animals are needed to assess the efficacy of tumor dye accumulation.

J. D. Steimle1, M. Park1, F. J. Grisanti Canozo1, S. Fang3, Z. A. Kadow1, T. T. Tran1, P. G. Swinton2, Y. Huang3, M. H. Samee1, J. F. Martin1,2 1Baylor College Of Medicine,Molecular Physiology And Biophysics,Houston, TX, USA 2Texas Heart Institute,Houston, TX, USA 3Institute of Biosciences and Technology, Texas A & M University,Center For Epigenetics & Disease Prevention,Houston, TX, USA
Introduction:
Atrial fibrillation (AF) is the most common sustained arrhythmia in the United States with a 25% lifetime risk, and accounts for one-third of all cardiovascular diseases. Treatment and care associated with AF costs roughly $26 billion/year in the US alone and accounts for 10% of all Medicare spending. Debilitating complications linked to AF include heart failure and stroke. AF risk is resoundingly linked to common variation at the 4q25 locus, implicating cis-regulation of the transcription factor PITX2. PITX2 is expressed in left atrial (LA) myocardium where it has been implicated as underlying AF phenotypes in both patients and animal models. Although animal models of Pitx2 are susceptible to AF, the direct mechanisms regulated by Pitx2 remain undetermined. To understand the molecular drivers of AF, it is imperative to understand both the upstream regulation and downstream targets of PITX2.
Methods:
Using Pitx2 gene-edited mice, we have performed a compendium of single nuclei RNA sequencing (snRNA-seq) and single nuclei assay for transposase-accessible chromatin sequencing (snATAC-seq) experiments to identify the role of Pitx2 in LA myocardium.
Results:
Using the snRNA-seq and snATAC-seq datasets, we identified differentially expressed genes with differentially accessible chromatin sites in both the cardiomyocyte and fibroblast populations of the LA. Among the genes in the LA cardiomyocytes, we see a reversal of left and right atrial expressed genes.
Conclusion:
AF is a complex genetic disease with many underlying genetic and physiological variables. In this work, we have begun laying the groundwork to understand the direct molecular mechanisms governed by Pitx2 in the LA myocardium. In future work, we hope to investigate the cis-regulatory mechanisms of PITX2 at candidate loci identified in this study. Additionally, we plan to identify the molecular machinery by which PITX2 interacts to govern the cis-regulatory landscape of the LA myocardium in development and disease.
A. Lam1, O. Zaborina1, J. Alverdy1 1University Of Chicago,Department Of Surgery,Chicago, IL, USA
Introduction:
Recent advancements in endoscopic surgery have spurred a rise in endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD) for the treatment of early stage esophageal malignancy. Complications from EMR/ESD include stricture formation, full thickness injury, delayed perforation, and leak. Therefore a more complete understanding of the mechanisms that govern esophageal epithelial regeneration following injury is needed to prevent the occasionally lethal complications. Here, we describe a simple model for culturing whole esophageal explants long term, providing a platform for potentially studying regeneration ex vivo.
Methods:
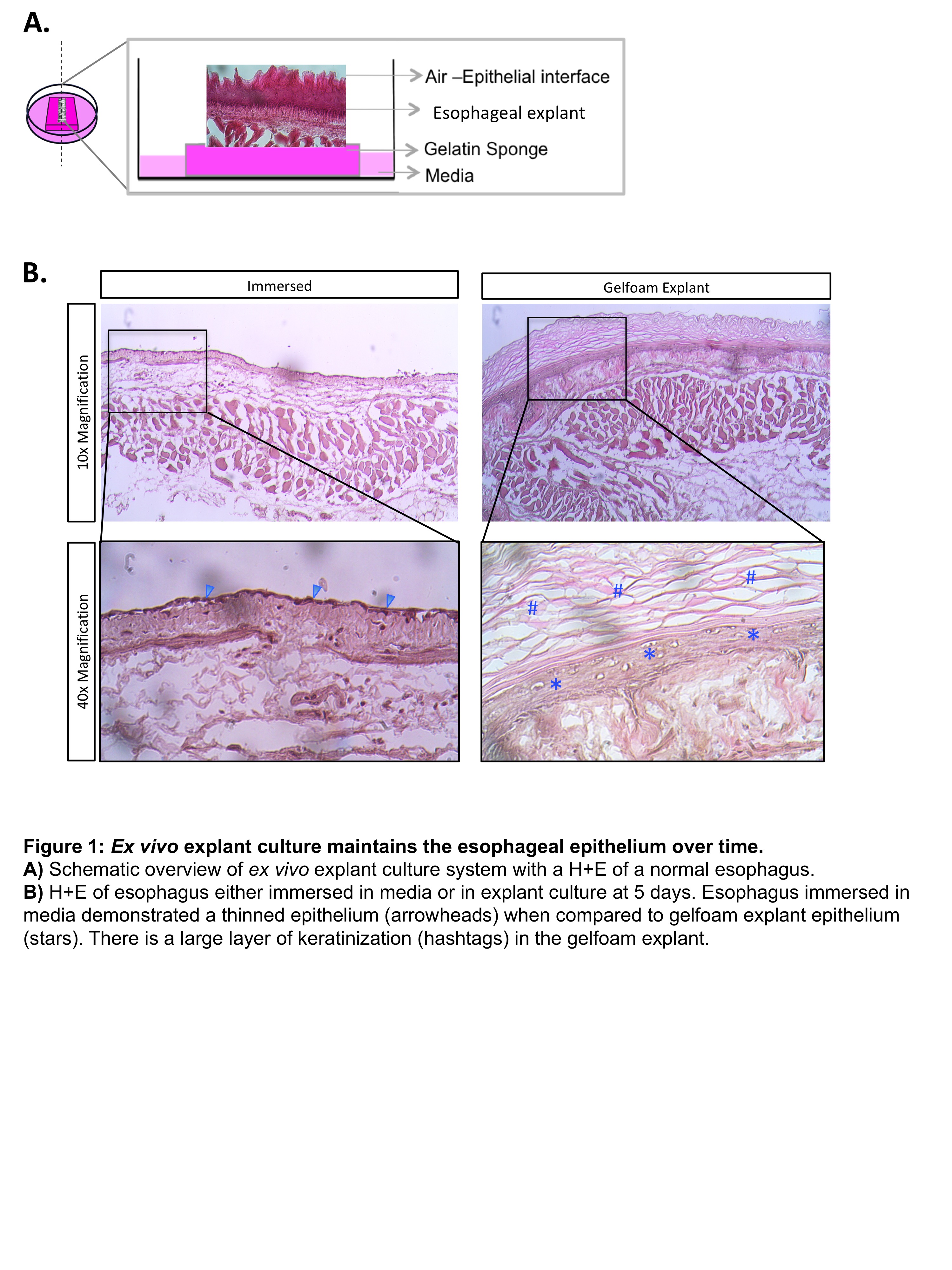
C57/BL6 wildtype mice were sacrificed and underwent sternotomy and laparotomy. The esophagus was dissected, opened longitudinally, and then either completely immersed in media or transferred atop a gelfoam sponge impregnated with media (DMEM/F12 with HEPES and Penicillin/Streptomycin). Following culture, tissues were fixed and cryosectioned in standard fashion. H&E staining was used to analyze explant architecture.
Results:
After the esophagus is completely immersed in culture for 5 days, there is loss of normal esophageal architecture characterized by atrophy of the esophageal mucosa. In contrast, when the esophagus is cultured atop a gelfoam sponge (mimicking an air-liquid interface), mucosal architecture is maintained. Furthermore, these explants also showed signs of mucosal proliferation, as evidence by an enlarged zone of keratinization. Additional studies demonstrated that this novel culture platform was able to maintain esophageal explants for up to 17 days without significant distortion of architecture.
Conclusion:
Long term whole organ esophageal explants can be maintained without compromising tissue integrity. This platform provides a novel yet simple method for potentially studying the process of esophageal regeneration following both superficial (i.e EMR) and deep (ESD) simulated injury in the mouse.
C. Audu1, S. Wolf-Fortune2, W. J. Melvin1, F. Davis1, A. DenDekker2, S. Sharma1, K. Mangum1, E. Barrett1, A. Joshi2, A. Obi1,2, B. Moore2, K. A. Gallagher1,2 1University Of Michigan,Vascular Surgery,Ann Arbor, MI, USA 2University Of Michigan,Microbiology & Immunology,Ann Arbor, MI, USA
Introduction: CD4+T-cells are vital for normal wound repair but the factors that control T-cell activation and plasticity in wounds in vivo are not clear. Our group and others have found increased Th17 activation in diabetic wounds results in increased IL17a and pathologic inflammation that prevents tissue repair. Plasmacytoid dendritic cells (pDC) are antigen presenting cells (APCs) that are present in early diabetic wound tissue and may play a key role in modulating CD4+T cell phenotype. Hence, we hypothesized that pDCs in diabetic wounds may activate CD4+ T-cells towards a Th17 phenotype.
Methods: Wild type C57BL/6 mice were fed normal chow diet (13.5% kcal fat) or high fat diet chow (60% kcal fat) for 12-14 weeks to generate the diet-induced obesity (DIO) model of glucose intolerance/insulin resistance. These mice were subsequently wounded with 6 mm punch biopsies, and wound pDCs were examined by flow cytometry daily for 5 days. In another cohort of mice, wounds were created and pDCs were harvested on day 1. These cells were co-cultured with wild type, naïve CD4+ T-cells for 5 days, after which T-cell phenotype was determined by flow cytometry for established transcription factors and extracellular markers.
Results: Following exposure to DIO pDCs, wild type activated CD4+ T-cells were polarized towards a Th17 phenotype, demonstrating increased I17a production. Further, DIO pDC recruited to wounds resulted in decreased Th2/IL-4 producing T-cells, that are important for normal wound repair. Further, there were kinetic differences seen in pDC recruitment to DIO versus control wounds following injury.
Conclusion: Diabetic pDCs play an important role in wound Th17 CD4+ T-cell activation and may act to increase inflammation in diabetic wounds.
T. A. MacArthur2, M. Auton3, A. Tischer3, J. Dong4, R. A. Kozar5, J. Goswami2, M. S. Park1 1Mayo Clinic,Department Of Surgery, Division Of Trauma, Critical Care And General Surgery,Rochester, MN, USA 2Mayo Clinic,Department Of Surgery,Rochester, MN, USA 3Mayo Clinic,Division Of Hematology, Department Of Medicine,Rochester, MN, USA 4University Of Washington,Hematology Division, Department Of Medicine, Member- Bloodworks Research Institute,Seattle, WA, USA 5University Of Maryland,Department Of Surgery,Baltimore, MD, USA
Introduction: Von Willebrand factor (VWF) is synthesized in megakaryocytes and endothelial cells (EC) as a single-chain propolypeptide that consists of repeat domains of D1-D2-D’-D3-A1-A2-A3-D4-C(1-6)-CK. After synthesis, VWF multimers are either released constitutively into circulation or stored in granules of ECs and platelets, where they form ultra-large (UL)VWF multimers. Under physiologic conditions, ULVWF is rapidly cleaved by the metalloprotease ADAMTS-13, up release, to generate the smaller plasma forms of VWF multimers found in circulation. This cleavage prevents spontaneous pro-thrombotic VWF-platelet interaction in circulation while maintaining the hemostatic activity of VWF at the site of vascular injury. After trauma, ULVWF multimers are released as part of the acute phase reaction. When ULVWF release overwhelms the proteolytic capacity of ADAMTS-13 this safeguard is bypassed, potentially contributing to the coagulopathy of trauma.
Methods: Fifty plasma samples from 30 trauma patients were evaluated and compared to 21 healthy volunteers. The patients were stratified by injury type including traumatic brain injury (TBI), Long Bone Fracture (LBFx) and need for any blood transfusion (TX). We assessed the plasma concentrations of VWF:Ag and ADAMTS-13:Ag, and the Rapid Enzyme Assays for Autoimmune Diseases (REAADS) activity of VWF, which measures the binding of a monoclonal antibody to an exposed epitope in the A1 domain. Kruskal- Wallis test for significance was performed on all subgroups, and results are presented in Table 1 against previously obtained thrombin generation data for the same patients.
Results: As compared to healthy volunteers, thrombin generation is enhanced in trauma patients, especially those that required any blood transfusion (Table 1). In all trauma patients, the VWF:Ag levels were elevated relative to controls. In addition, the REAADs activity was greater, especially in those with TBI. ADAMTS-13:Ag levels were also moderately reduced in all trauma patients, and this was most apparent in those with TBI.
Conclusion: These data demonstrate that trauma patients, especially those with TBI, have enhanced exposure of the VWF A1 domain (activated VWF) based on increased REAADS activity. The increase in REAADS activity coupled with the decrease in free plasma ADAMTS-13:Ag in all trauma patients, may indicate that ADAMTS-13 is not efficiently cleaving ULVWFs in these patients. Further investigation with a larger cohort is merited to assess whether VWF has any clinical significance in Trauma-induced Coagulopathy, such as in the development of venous thromboembolism.
A. M. Jacques1,2, M. Crawford1,2, G. M. McCaughan1,2, C. Pulitano1,2 1Royal Prince Alfred Hospital,Transplantation Surgery,Sydney, NSW, Australia 2University of Sydney,Faculty Of Health And Medicine,Sydney, NSW, Australia
Introduction:
The shortage of quality donor organs has resulted in the widespread use of sub-optimal livers in clinical transplantation. Marginal grafts are particularly susceptible to injury after procurement during the period of static cold storage. Normothermic machine perfusion (NMP), where metabolic activity is maintained, is a potential alternative to impove the preservation of marginal grafts. If the safe preservation of metabolically active grafts can be extended for multiple days, an opportunity arises to introduce therapeutic agents and further optimise these organs. The accumulation of metabolic waste, however, limits the current utilisation of NMP beyond 24 hours. It is the aim of this study to evaluate the technical feasibility of prolonged (5-day) normothermic perfusion of discarded liver grafts with modifications to our current NMP system.
Methods:
Six discarded human liver grafts were perfused for 120 hours after the development of a newly adopted perfusion system incorporating: 1) long-life oxygenators; 2) a dialysis membrane; and 3) continuous parenteral nutrition. Perfusate volume consisted of packed red blood cells, fresh frozen plasma, concentrated albumin and crystalloid. In addition to homogenous perfusion and stable vascular flows, the viability of the grafts was assessed at 4 hours, and then every 24 hours for five days using: 1) maintenance of pH; 2) lactate <2.5mmol/L; 3) production of bile; and 4) biliary pH >7.4. Hepatocellular injury, protein synthesis, and clearance of water-soluble by-products were assessed using alanine transferase (ALT), coagulation factor V and urea levels respectively.
Results:
Five of six livers met all viability criteria after 72 hours, and three of six at 120 hours. One organ failed to reach viability criteria at all time points. Two livers initially failed to meet criteria due an inability to produce alkaline bile that that resolved over the perfusion duration. Five of six livers demonstrated a down-trending ALT after a peak within 24 hours. Initially low levels of factor V increased to >75% in five of six livers during perfusion. Clearance of water-soluble metabolic by-products was evident by a stable urea concentration from day 1 to 5 across all grafts. Macroscopic pressure necrosis was evident to varying extents in all grafts at the conclusion of perfusion. Two grafts demonstrated macroscopic evidence of fungal infection.
Conclusion:
This study sucessfully demonstrates the technicaly feasibility of preserving metabolically active human livers for 5 days using NMP. The sensitivity of current real-time viability criteria, however, have significant limitations that create challenges in the assessment of therapeutic agents that may be introduced in the future. Prior to this approach transitioning to clinical transplantation the validation of alternative viability criteria and alternative strategies to overcome both pressure necrosis and infection must be overcome.
E. C. Barrett1, W. J. Melvin1, F. M. Davis1, C. Audu1, A. Kimball1, A. Joshi2, A. Obi1, K. Gallagher1,2 1University Of Michigan,Vascular Surgery,Ann Arbor, MI, USA 2University Of Michigan,Microbiology And Immunology,Ann Arbor, MI, USA
Introduction: Non-healing wounds in patients with Type 2 Diabetes (T2D) are a major cause of increasing morbidity and mortality. We and others have identified that a wound CD4+Treg cell phenotype is important for normal tissue repair, however the predominant CD4+ T cell phenotype in T2D wounds is unclear. Thus, we hypothesized that in diabetic wounds Tregs are decreased, while a TH17 phenotype predominates and serves to promote excess inflammation and impairs healing.
Methods: Using a murine wound healing model, wound Mφs were analyzed by flow cytometry from control and diet-induced obese (DIO) mice (a model of T2D) and FoxP3EGFP reporter mice (n= 40/group). Human T2D and non-T2D wounds were isolated and single cell RNA sequencing was performed. Statistical significance was determined using Student’s t-test or ANOVA.
Results: Using our FoxP3EGFP reporter mice, we have identified that in normal wound repair, FoxP3+CD4+Tregs are increased and TH17 cells are decreased on day 5, during the resolution phase of normal tissue repair. In contrast, in diabetic wounds, Tregs are decreased and TH17 are increased on day 5. Further, we found increased IL17A production by diabetic CD4+T cells compared to control wounds. Single-cell transcriptional profiling of human diabetic wounds revealed increased RORγt+CD4+T cells (TH17) and decreased FoxP3+CD4+Tregs.
Conclusion: These results identify that TH17 cells are increased in murine and human diabetic wounds and may contribute to the excess inflammation in diabetic wounds that prevents healing.
A. Akil1, V. G. Bolus1, A. W. Beck1, H. Chen1, B. Ren1 1The University of Alabama at Birmingham,Surgery,Birmingham, ALABAMA, USA
Introduction: Pancreatic neuroendocrine tumors (PNETs) are highly vascularized and heterogeneous neoplasms, which are characterized by high levels of VEGF and its receptors, the potential driver in PNET metastasis. An antiangiogenic drug known as sunitinib has been approved for the treatment of PNETs. However, the therapeutic efficacy is limited because of relapse and metastasis. Therefore, tailoring antiangiogenic therapy to patients requires novel insight of tumor angiogenesis. Our previous studies suggested that PKD-1 signaling stimulates arteriolar differentiation (a key process in functional angiogenesis) via the Notch pathway and promotes tumor progression. We hypothesize that PKD-1-stimuated arterial differentiation plays an essential role in PNET progression by development of a unique vascular niche.
Methods: To test this hypothesis, we developed a co-culture system for growing human microvascular endothelial cells (HMVECs) with PNET cells using a Boyden Chamber technique. To define the molecular mechanisms by which PKD-1 signaling regulates PNET progression, we knocked down endogenous PKD-1 expression and characterized malignant behavior of PNET cells using Clonogenic and tumor invasion assays. Furthermore, real-time RT-qPCR was used to evaluate the transcriptional expression of genes associated with arteriolar differentiation and tumor progression.
Results:Compared with the scramble control, PKD-1 knockdown in HMVECs decreased the formation of colonies and impaired the invasive potential of PNET cells. Unexpectedly, co-culture of control HMVECs with PNET cells showed clear formation of many colonies with tumorsphere-like morphology. However, when these cells co-cultured with PKD-1-deficient HMVECs, the number of spheres was significantly reduced. At molecular levels, knocking down PKD-1 in HMVECs reduced mRNA expression of ALDH1A1, VEGFR2 and Notch1 but increased CD36 expression when co-cultured with PNET cells. Intriguingly, silencing PKD-1 in HMVECs downregulated mRNA levels of KLF2, Myc, ALDH1 and Snail 1 (a transcriptional factor for epithelial mesenchymal transition) in PNET cells.
Conclusion:This study indicates that there is a crosstalk between endothelial cells and PNET cells to promote arteriolar differentiation, in which PKD-1 signaling may play a key role. Cancer cell-mediated arteriolar differentiation via the PKD-1 pathway may contribute to the progression of PNETs by serving as a unique vascular niche in the tumor microenvironment.
K. Khabaz1, S. Dhara3, C. J. Lee4, R. Milner2, L. Pocivavsek2 1University of Chicago,Chicago, ILLINOIS, USA 2University of Chicago Pritzker School of Medicine,Department Of Vascular Surgery,Chicago, ILLINOIS, USA 3University of Chicago,Pritzker School Of Medicine,Chicago, ILLINOIS, USA 4NorthShore University HealthSystem,Department Of Surgery,Chicago, ILLINOIS, USA
Introduction:
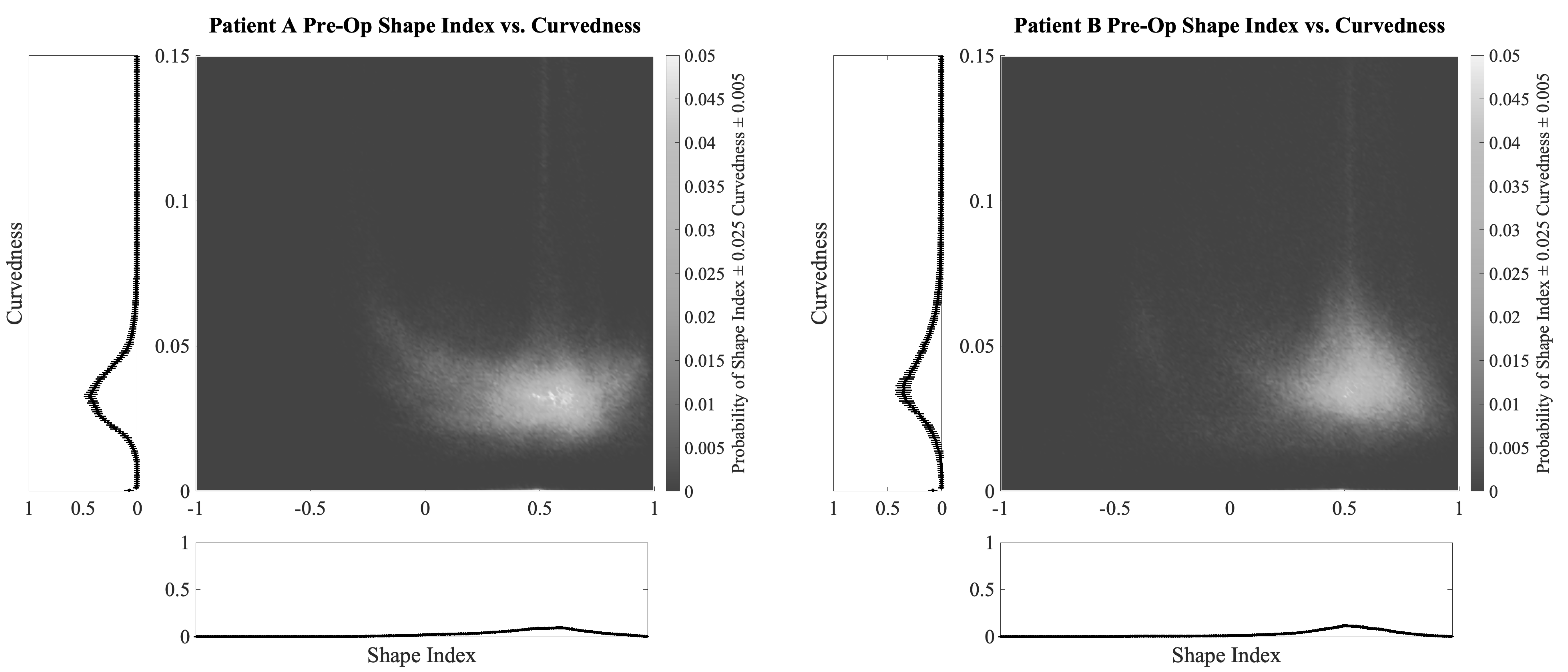
Type B aortic dissections (TBADs) have historically been treated with surgery or medical management, depending on the presence of complications. The advent of thoracic endovascular aortic repair (TEVAR) has necessitated a reconsideration of the treatment paradigm due to its reduced peri-operative risk profile but significant long-term complication rate. The guidelines regarding TEVAR versus open surgery still remain in flux and will require greater clarification in the future, particularly with regards to the best choice to reduce complications. We hypothesize that accurate guidance in choosing between endovascular and open repair can be provided by computational characterization of aortic surface geometry, specifically using two indices of surface curvature: the Koenderink shape index, which measures shape, and curvedness, which measures the intensity of the shape. Here, we demonstrate our method of analysis by comparing two TBAD patients with similar non-complicated dissections but distinct post-operative outcomes.
Methods:
We study two TBAD patients (A and B) with non-complicated dissections but two distinct post-operative clinical outcomes: aortic remodeling with false lumen thrombosis and failed remodeling with post-procedural complications (type 1A endoleak). Utilizing CTA imaging, pre- and post-op segmentations of the aortic wall are performed. The curvature tensor is calculated for a mesh of each geometry, allowing us to measure the principle curvatures (k1,k2). Using the Koenderink shape index, s = 2/π arctan((k1+k2)/(k1-k2 )), and curvedness, c = √((k1+k2)^2/2), we measure characterize aortic shape by plotting "2D" histograms of s versus c over each meshed aortic surface. We then calculate statistics of each distribution.
Results:
The figure summarizes our results comparing patient A and B. Although the mean shape index, mean curvedness, and variance in shape index values are similar (0.475 vs. 0.481, 0.0352 vs. 0.0412, 0.0640 vs. 0.0641), patient A has a smaller variance in curvedness than patient B (0.00194 vs. 0.000341). This is visually reflected by the larger vertical spread of high intensity regions for patient B.
Conclusion:
Despite relatively similar presentation of non-complicated dissections, the two patients in this study experience different outcomes. Computing the shape index and curvedness values provides a novel and more comprehensive view of the surface geometry. We saw that the variance in curvedness was able to distinguish between a patient with positive post-operative outcomes and a patient with post-operative complications. We hope to determine if our method of analysis holds predictive power with a larger patient set.
E. Lopez1, L. Wood1, I. Marques1, L. Theiss1, C. Shao1, R. Hollis1, J. Cannon1, M. Morris1, D. Gunnells1, G. Kennedy1, K. Hardiman1, D. Chu1 1University Of Alabama at Birmingham,Birmingham, Alabama, USA
Introduction: Patients with limited health literacy have difficulty understanding health information, which may lead to worse health outcomes. As telehealth use increases in surgery, especially as a response to COVID-19, communication increasingly relies on conversation. It is unclear, however, if variations exist in these conversations that may be amenable to improvement. This study aimed to understand the relationship between patient and physician speech on understandability in patient-surgeon telehealth encounters.
Methods: New and returning telehealth surgical patients for six surgeons at the UAB Colorectal Clinic (June-July 2020) were recruited and consented. Health literacy was measured using the Brief Health Literacy Screening Tool (BRIEF). Patient-surgeon conversations were audio-recorded during telehealth visits. Recordings were transcribed and analyzed for questions asked, length of visit (LOV), and understandability of words by the Flesch-Kincaid Ease Score (FKES), Flesh Kincaid Grade Level (FKGL), Gunning-Fog Index (GFI), Coleman-Lau Index (CLI), and Simple Measure of Gobbledygook (SMOG).
Results:Twenty-four patients were enrolled. The mean age was 49.5 years (+/- 12.7) and 66.7% were female. Health literacy levels were adequate (11 patients, 45.8%), marginal (10 patients, 41.7%) and low (3 patient, 12.5%). Speech between surgeons varied by understandability and LOV. Overall average LOV was 10 minutes and 3 seconds (+/- 6 minutes and 40 seconds) among all surgeons (N=24). Overall average SMOG score was 5.2 (+/-1.2) and overall average FKES was 84.3 (+/-6). Lower understandability was associated with longer LOV (p<0.0003), longer physician speaking time (p<0.0003), and more words spoken by physician (p<0.0009). However, higher percentage words spoken by patient was associated with higher understandability (p<0.01).
Conclusion:Length of visit and understandability vary among surgeon speech during patient-surgeon telehealth encounters. Longer physician speaking time is significantly associated with less understandable words while more speaking time for patients is associated with more understandable encounters. Creating an environment where patients with low health literacy are able to speak more and ask more questions may help improve patient understanding.
L. Theiss1, T. Wood1, I. Marques1, C. Shao1, R. Hollis1, K. Hardiman1, D. Gunnells1, J. A. Cannon1, M. S. Morris1, G. D. Kennedy1, D. I. Chu1 1University Of Alabama at Birmingham,Department Of Surgery,Birmingham, Alabama, USA
Introduction: Patient engagement technologies (PETs) are novel technologies that guide patients through the surgical journey, reduce costs of care and may lead to improved surgical outcomes. However, not all patients choose to enroll in a PET and the reasons are unclear. This study examines if patient demographics or insurance status are associated with patients declining to enroll in a PET.
Methods: Patients undergoing elective colorectal, thoracic, cardiac, and gynecological oncology surgery at a single institution were approached about enrolling in a PET (SeamlessMD) at their preoperative clinic visit. For patients who enrolled, healthcare reminders, educational content, PRO surveys, and health checks were distributed via the PET preoperatively, in-hospital, and for up to 30 days post-discharge. Patients who decided not to enroll were recorded and went through usual surgical care. Patient age, sex, race, and insurance status were compared between patients who elected to enroll in the PET at the initial clinic visit and patients who declined to enroll in the PET. Groups were compared using Wilcoxon signed rank test and independent t-test with alpha value of 0.05 determined a priori.
Results: Between 2018-2020, 1699 patients were approached about enrolling in the PET. 1609 (94.7%) elected to enroll, while 90 (5.3%) declined. Median age varied significantly between the groups, with median age of 60 for patients who enrolled compared to 66 for those who refused (p<0.001). Insurance status also varied significantly. Of patients who enrolled, 47.3% had Medicare/Medicaid, 41.8% were privately insured, and 10.9% were uninsured or charity care. Of patients who declined, 78.7% had Medicare/Medicaid and 21.4% of patients were privately insured (p<0.001). Patients who refused were more likely to be Black or African American, however this difference was not statistically significant (37.5% vs 30.7% for enrolled patients, p=0.24). There was no difference in PET enrollment by sex.
Conclusion: Older patients and patients who are not privately insured are more likely to decline PET enrollment. While PETs can be useful technology for navigating the surgical journey, they may not be universally adopted or provide benefit to all patients. Considerations should be made for complementary or alternative resources for these non-PET patients.
R. Z. Strigenz1, P. B. Schwartz1, C. C. Stahl1, T. J. Aiken1, A. W. Acher1, E. H. Lawson2, D. Kravtsov1, P. R. Carney1, D. E. Abbott1 1University Of Wisconsin,Surgical Oncology,Madison, WI, USA 2University Of Wisconsin,Division Of Colorectal Surgery,Madison, WI, USA
Introduction: Patients living in geographically rural areas have increased barriers to healthcare access, including cancer care. Relative to urban populations, patients living in rural areas have an increased incidence of cancer and reduced survival, among other disparities. We sought to determine whether patient rurality was a driver of hospital cost for patients undergoing colectomy for colon cancer.
Methods: All patients undergoing colectomy for primary colon cancer at an academic medical center were queried from 2014 to 2019. Institutional cancer and cost accounting databases were combined, and patients were stratified into urban (1-3) and rural (4-10) cohorts by rural-urban commuting area (RUCA) codes. Bivariate analysis comparing the rural-urban cohorts and index hospitalization cost was conducted with student’s t-test, ANOVA, linear regression, and Kaplan Meier log rank analysis as appropriate. Relevant variables were entered into a multivariable linear regression to identify pertinent drivers of hospital cost. Significant variables on multivariable linear regression were reported as the beta (change in $ per unit change) with an associated 95% confidence interval.
Results:
279 urban and 142 rural patients were included in the final analysis. Rural patients were more likely to be non-Hispanic white (p=0.02), have lower alcohol use (p=0.03), differ in their insurance payor (p=0.02), undergo an open operation (p<0.01), be further from the treating institution (p<0.01), and have a worse median recurrence free survival (23.1 months vs. 27.1 months, p=0.04). On bivariate analysis, no differences in hospital cost was found between urban and rural patients ($12,151.16 vs. $13,082.61; p=0.42).
These findings were affirmed on multivariable linear regression analysis ($484.84; CI -1,699.69-2,637.38; p=0.67) after controlling for patient-related factors including age, race, rurality, insurer and ASA class, tumor-related factors including TNM staging and grade, and operative factors including operative time, open vs. laparoscopic procedure, and type of colectomy performed. Instead, on multivariable analysis (Table 1), statistically significant drivers of hospital cost included ASA class, T stage, operative time, and operation performed.
Conclusion: While differences between rural and urban patients undergoing surgical treatment for colon cancer exist, our results demonstrated no association between rurality and hospital cost. This persisted with multivariable analysis, suggesting that although patient rurality may affect various aspects of cancer care, institutions are able to provide the same high value care at costs similar to urban patients.
S. Bueno Motter1, J. Iaroseski1, G. Rangel Brandão1 1University of Health Sciences of Porto Alegre,Porto Alegre, RIO GRANDE DO SUL, Brazil
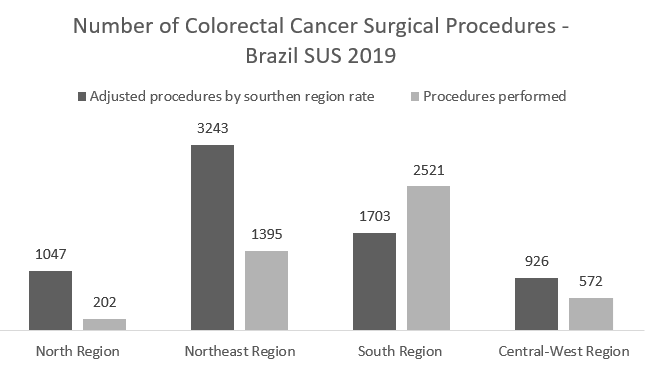
Introduction: The global burden of colorectal cancer (CRC) is an issue that needs attention, especially in low- and middle-income countries. In Brazil, in 2018, in male population, among all new cases of cancer, CRC was the third most common. In the female population, CRC was the second most common. As there are disparities of health care among high-, low-, and middle-income countries, also there are disparities within the countries. Therefore, this study aims to verify, among the five geographic regions of Brazil, the disparities of estimation of new cases of CRC for 2020 and the surgical treatments for this tumor performed in 2019 by Unique Health System (SUS).
Methods: We analyzed the estimation data of new cases of CRC for 2020 from the National Cancer Institute (INCA) and the data of surgical treatments for this tumor performed by Unique Health System (SUS) in 2019. We compared the data of North, Northeast, South, and Central-West Regions to the data of the Southeast Region, which is the most developed region in Brazil, to verify among these regions the possible differences of estimation of new cases of CRC and surgical treatments. To base this analysis we used the estimation of Brazilian population for 2019 from Brazilian Institute of Geography and Statistics (IBGE).
Results: With a population of 88 million inhabitants and a Human Development Index (HDI) of 0,753, Southeast Region has an estimation of 24.260 new cases of CRC for 2020 (INCA). For North Region, this estimation is 1100 new cases (18 million inh.; HDI 0,690), Northeast is 5760 (57 million inh.; HDI 0,658), South is 7370 (29 million inh.; HDI 0,756) and Central-West is 2520 (16 million inh.; HDI 0,753). If the estimation of new cases were proportionally the same as the Southeast, which is the region of greatest socio-economic relevance in Brazil, the North region would have an estimation of 5060 new cases, 360% more than the present estimation. In the Northeast region there would be 15667 new cases, 172% more than the current estimation. For surgical treatments performed by SUS, if the North Region had the same rate as the Southeast Region, there would be an increase of 418% in surgical treatments, in Northeast the increase would be 132%, and in Central-West there would be an increase of 62%. The only region that proportionally performed more surgeries than Southeast was South Region.
Conclusion: There is a significant disparity among regions of Brazil concerning estimation of new cases of CRC and surgical treatments. If all Brazilian population had the same treatment and diagnostic screening rates as Brazilian Southeast population has, more inhabitants would have access to health care and the burden of cancer could be reduced.
V. M. Welten1,2, K. Wanis3, A. C. Fields1, P. W. Lu1, R. A. Malizia1, J. Yoo1, J. E. Goldberg1, J. L. Irani1, R. Bleday1, N. Melnitchouk1,2 1Brigham And Women’s Hospital,Division Of General And GI Surgery, Department Of Surgery,Boston, MA, USA 2Brigham And Women’s Hospital,Center For Surgery And Public Health,Boston, MA, USA 3Harvard School Of Public Health,Department Of Epidemiology,Boston, MA, USA
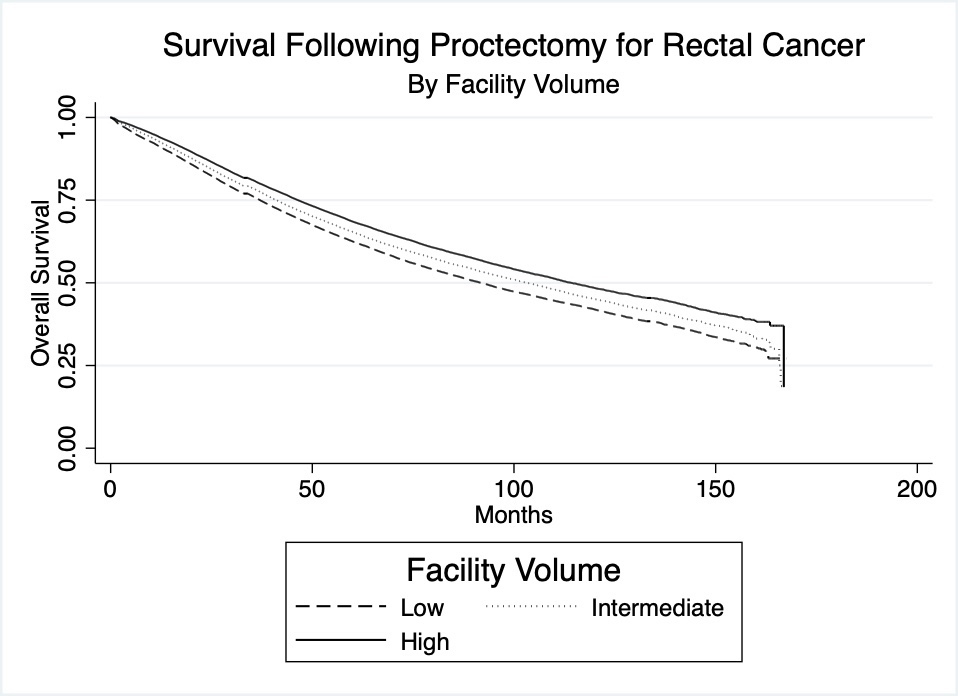
Introduction: The management of rectal cancer has become progressively more complex, requiring a multi-disciplinary approach. Centralization of care has been increasingly discussed as a means to improve outcomes following high-risk cancer surgery. Prior studies assessing survival of patients with colorectal cancer have reported better outcomes when operations are performed at high-volume centers, with both lower complication rates and lower mortality rates, while others have suggested that differences in outcomes are related to degree of surgeon specialization rather than case volume. We aim to examine the association between facility volume and survival following proctectomy for rectal cancer.
Methods: The National Cancer Database was used to identify all patients who underwent proctectomy for rectal cancer between 2004-2016. Facility volume was determined by the frequency of proctectomy for rectal cancer based on a facility’s unique ID, and was divided into tertiles – low, intermediate, and high. Median overall survival and 5-year survival for the patient cohort was compared between facility volume tertiles. Log-rank tests and Kaplan-Meier survival statistics were used for comparison.
Results: A total of 158,764 patients were included. Facilities were divided into low, intermediate, and high volume tertiles. When analyzing facility volume by facility type, for high volume facilities, 57% were academic and 38% were non-academic; for low volume facilities, 11% were academic, while 86% were non-academic (p<0.001). Median survival was 91.9 months (95%CI 90.1 – 93.5) for low volume facilities, 103.2 months (95%CI 101.3 – 105.5) for intermediate volume facilities, and 114.2 months (95%CI 111.9 – 116.7) for high volume facilities, with differences between tertiles statistically significant (p< 0.001). 5-year survival rates for the three tertiles were 62.5% (95%CI 62.0 – 63.0) for low volume, 65.4% (95%CI 64.9 – 65.8) for intermediate volume, and 68.5% (95%CI 68.1 – 69.0) for high volume (p<0.001). The corresponding Kaplan-Meier graph is presented in Figure 1: Overall survival following proctectomy for rectal cancer by facility volume.
Conclusion: This study identifies a strong association between facility volume tertile and survival following proctectomy for rectal cancer. Further work is needed to evaluate drivers of this association, whether there is a causal effect of facility volume on survival, and whether this relates to specialist expertise, compliance with treatment guidelines and delivery of standard of care, or other factors.
O. Olaleye1, I. Marques1, L. Theiss1, C. Shao1, S. Bergstresser1, K. Hardiman1, D. Gunnells1, J. Cannon1, G. Kennedy1, M. Morris1, D. Chu1 1University Of Alabama at Birmingham,Division Of Gastrointestinal/Department Of Surgery,Birmingham, Alabama, USA
Introduction: According to the 2003 National Assessment of Adult Literacy (NAAL), 9 out of 10 U.S. adults have difficulty reading and understanding health information. Hospital discharge materials contain tremendous amounts of health information, but it is unclear if these materials are readable, understandable and actionable. The aim of this study was to assess surgical discharge materials for these parameters with the hypothesis that discharge materials are not readable, understandable or actionable.
Methods: Discharge materials for a colorectal surgery service were collected from a single-institution. Readability, understandability and actionability were assessed by 3 independent reviewers using validated and complementary instruments: (i) Flesch-Kincaid Grade Level (FKGL), (ii) Gunning-Fog Scale (GF), (iii) Coleman-Liau Index (CL), (iv) Simple Measure of Gobbledygook (SMOG), (v) Automated Readability Index (AR), (vi) Linsear Write Formula (LW), (vii) Flesch Reading Ease (FRE), (viii) Patient Education Materials Assessment Tool (PEMAT), and (ix) Print Communication Rating (PCR). Scores were averaged and compared across discharge materials to identify areas for improvement.
Results: 79 discharge items covering 131 pages were collected for analysis. On readability assessments, discharge materials were mostly written below the 8th grade reading level by all readability tests except the GF (9.82 ± 0.44) and CL (9.67 ± 0.15). On assessment of understandability and actionability, discharge materials scored poorly including 55.92% ± 5.34% understandability and 42.18% ± 7.03 actionability by PEMAT (indicating readers’ difficulty to process key points and identify their role within the information presented) and 73.24% ± 3.10 PCR (indicating adequate presentation of information or directions but in need of improvement). Lowest scoring domains were opioid pain medication instructions (9.8 GF, 10.1 CL, and 60.3 FRE), laparoscopic colectomy information (11.5 GF, 10.4 CL, and 62.8 FRE), and forms on topics ranging from tobacco use to a health app (10.5 GF, 11.5 CL, and 59.8 FRE).
Conclusion: While most discharge materials were written below the 8th grade reading level, major opportunities exist for improving their understandability and actionability. Key areas of improvement include using more visual aids, simplifying content, and providing examples for medical terminology. Future studies will focus on improving discharge materials to make them more understandable and actionable.
S. Wang1,2, H. Huang1, E. Scheufele1, I. Dankwa-Mullan1, G. P. Jackson1,2, Y. Arriaga1 1IBM Watson Health,Cambridge, MA, USA 2Vanderbilt University Medical Center,Nashville, TN, USA
Introduction: Time to surgery has been a well-known quality metric for colon cancer management, with evidence supporting improved clinical outcomes for patients who receive prompt versus delayed surgical therapy. We investigated trends and patient-related factors associated with time to surgery in a cohort of insured patients with colon cancer.
Methods: A cohort was identified from the IBM® MarketScan® database from January 2013 to December 2018. Inclusion criteria entailed patients 18 years and older with an initial diagnosis of non-metastatic colon cancer undergoing surgery within 6 months of diagnosis, with continuous insurance enrollment from 12 months pre- to 6 months post-diagnosis. Claims for surgery within six months after diagnosis were evaluated. Temporal trends for time to surgery were estimated by the Kruskal-Wallis test. Logistic regression identified factors associated with time to surgery.
Results: In this study, 20450 patients with non-metastatic colon cancer had surgery within 6 months of diagnosis. Median age was 62 years (IQR = 20 years), and 9998 (49%) were female. Median time to surgery was 13 days. A statistically significant difference in time to surgery was found comparing 2013 (median 12 days) to 2018 (median 16 days) (p < 0.0001). Factors associated with undergoing expedited surgery (within 1 week of diagnosis) included: older age (p=0.05), female sex (p = 0.016), coronary artery disease (p=0.014), smoking (p < 0.0001), dementia (p =0.001), hemiplegia or paraplegia (p = 0.001), renal disease ( p=0.029), and resident of US South (p=0.012) or US Midwest (p=0.03). Conversely, male sex (p=0.027), residency outside of the US West (p=0.022), obesity (p<0.001), severe liver disease (p=0.011), and myocardial infarction (p=0.019) were associated with a higher likelihood of delayed surgery (greater than 1 month after diagnosis).
Conclusion: For patients with non-metastatic colon cancer, time to surgery demonstrated a significantly increasing trend from 2013 to 2018. Numerous demographic, clinical, and geographic factors were associated with expedited or delayed time to surgery. Identifying these factors may provide insights into the groups of patients at risk for delayed surgical treatment and could support interventions to optimize surgical outcomes and foster equitable delivery of surgical care.
N. P. Perez1, L. Bordeianou1, R. Ricciardi1, H. Kunitake1, C. Cauley1, J. Conley1, R. A. Hodin1, D. Berger1, J. C. Cusack1, C. E. Stafford1, C. W. Hunt1, G. M. Boland1 1Massachusetts General Hospital,Surgery,Boston, MA, USA
Introduction:
The perioperative journey is complex and difficult to navigate for patients and their families. The purpose of this study was to compare the outcomes after colorectal surgery between patients enrolled in a 1-year pilot of a technology-augmented ERAS pathway compared with historic controls enrolled in a paper-based pathway.
Methods:
Adult patients undergoing elective colorectal surgery from 6/11/2020–7/9/2020 were included in this study, as part of an ongoing 1-year pilot. Patients were enrolled on a commercially available patient engagement and data collection platform, which allows for the delivery of reminders, surveys, and educational materials created by our institution’s colorectal surgery service via a mobile app, text messages, and/or emails. Surveys delivered include patient-reported outcome (PRO) questionnaires and daily health checks meant to aid in the early detection of complications. The primary outcome was 30-day readmissions compared to historic controls. Secondary outcomes included preoperative anxiety, engagement and satisfaction with the platform. Patient anxiety was measured using the PROMIS-anxiety survey and patient engagement was ascertained by the completion of daily health checks and PRO surveys. Satisfaction with the platform was measured using the system usability scale (SUS) and the net promoter score (NPS).
Results:
A total of 40 patients were enrolled on the platform. Their mean age was 53.0 ±16.6 years old, 48.8% were female, 90% were White; 23 (57.5%) patients downloaded the mobile app. Compared to historic controls, patients using the platform had a lower rate of 30-day readmissions (10% vs 19%) and felt less nervous/anxious before surgery (11.4% vs 22.7% often/always). App users opened it an average of 2.9 times per day. Out of 280 daily health checks delivered, 187 (67%) were completed, with 86% of patients completing at least 3 of them. Postoperative PRO collection rates were 67.8% at 1 week, and 50% at 3 weeks, an increase from previous rates of approximately 10% using paper-based surveys. Most patients felt the platform was easy to use (69.2% agree/strongly agree) and its functions were well integrated (76.9% agree/strongly agree), with an overall NPS of 70%.
Conclusions:
The addition of a patient engagement and data collection technology platform to aid in the delivery of an existing ERAS pathway appears to have benefitted patient care, both with regards to outcomes as well as patient experience, satisfaction, and PRO collection. We expect the benefits of this technology to become even clearer as our 1-year pilot progresses, and we reach our projected total enrollment of 600 patients (50 additional have been enrolled since 7/9/2020). As mobile technologies become even more widespread and ubiquitous, it is incumbent upon us to adapt the way we deliver care and take advantage of these resources, to ultimately provide our patients with the outcomes and surgical experience they deserve.
P. E. Serrano1,3,4, C. Griffiths1, A. Gafni3, S. Parpia2,3,4, L. Linkins5, M. Simunovic1,2,3 1McMaster University,Surgery,Hamilton, ONTARIO, Canada 2McMaster University,Oncology,Hamilton, ONTARIO, Canada 3McMaster University,Department Of Health Research Methods, Evidence, And Impact,Hamilton, ONTARIO, Canada 4Ontario Clinical Oncology Group,Hamilton, ONTARIO, Canada 5McMaster University,Medicine,Hamilton, ONTARIO, Canada
Introduction:
Venous thromboembolism (VTE) occurs in approximately 10% of patients following major abdominal cancer surgery. Recent practice guidelines recommend the routine use of VTE prophylaxis for 28 days following surgery, typically extending prophylaxis beyond hospital discharge. We sought to characterize and compare awareness, agreement, adoption, and adherence to these guidelines among colorectal and hepatobiliary surgeons.
Methods:
We electronically surveyed Canadian hepatobiliary surgeons registered with the Canadian Hepatopancreatobiliary Association, and, general surgeons and colorectal surgeons registered with the College of Physicians and Surgeons of Ontario and the Canadian Society of Colorectal Surgeons, respectively, who provide care for patients with colorectal cancer with a pilot-tested questionnaire. Attitudes to relevant guideline recommendations and perceived barriers to post-discharge VTE prophylaxis were assessed on a 5-point Likert scale. Comparisons between specialties and attitudes towards guidelines were performed using 1-way ANOVA and Kruskal-Wallis tests.
Results:
There were 128 responses (response rate 60%, 128/213), including 60 general/colorectal and 68 hepatobiliary surgeons. Most surgeons (122 /128, 95%) were aware, agreed (101/122, 83%), adopted (78/101, 77%) and adhered (74/78, 95%) with post-discharge VTE prophylaxis guidelines[MOU1] [CG2] . Hepatobiliary surgeons, compared to surgeons performing colorectal cancer surgery were more likely to agree (94% vs. 69%), adopt (88% vs. 59%) and adhere (98% vs. 86%) with these guidelines. Insufficient evidence (median Likert: 4, IQR 3-5) and low incidence of VTE (median Likert: 4, IQR 3-4) were cited as the strongest barriers among respondents that did not agree with post-discharge VTE prophylaxis. Surgeons that agreed but did not adopt post-discharge VTE prophylaxis programs reported that the most significant barriers were cost (median Likert: 4, IQR 3-4), low support from surgical colleagues (median Likert: 4, IQR 4-4) and difficulty of subcutaneous injections (median Likert: 4, IQR 3-4), whereas surgeons that adhered additionally reported “logistical challenges of prescribing” as one of the greatest barriers to implementation.
Conclusion:
There remains apprehension regarding implementation of post-discharge VTE prophylaxis following abdominal cancer surgery among non-hepatobiliary surgeons, citing poor evidence and cost of the medication as the major barriers. Uptake among hepatobiliary surgeons versus surgeons performing colorectal cancer surgery was higher.
C. Varlamos1, C. Hibbard1, S. J. Rivard1, A. Duby1, M. Callow1, J. Dimick1, J. Byrn1, M. Byrnes1 1University of Michigan Medicine,Department Of Surgery,Ann Arbor, MI, USA
Introduction:
The role of vulnerability has been widely discussed as a requirement for continuous professional development. Yet, we understand little about this concept in surgeons. Given the nature of surgery, which values confidence, it remains challenging for surgeons to embrace vulnerability. This study explores surgeons’ experiences and views on vulnerability in the context of a group surgical coaching intervention.
Methods:
Data for this study is part of an ongoing longitudinal qualitative study exploring expert learning among 14 Michigan surgeons participating in a surgical coaching program. For a more diverse perspective, we conducted one-on-one in-depth interviews with an additional 68 colorectal surgeons across the U.S. to gauge attitudes and perceptions around surgical coaching. The 82 interviews were analyzed in a team-based approach and results are reported thematically.
Results:
Surgeons reflected on three main themes surrounding vulnerability including technical, social, and the importance in continuous professional development. Participants described coaching as an event that may situate the surgeon as technically vulnerable. Technical vulnerability relates to reluctance to adapt to new surgical techniques, fear of displaying techniques to peers, and knowledge about challenging cases. Surgeons also discussed social vulnerability or threats to a surgeon’s professional identity. Participating surgeons were critical of their mannerisms during coaching sessions and cognizant of the social hierarchy of surgeon “rock stars” in the room. For non-participants, many suggested that social vulnerability may be related to intersectional identities such as race, class, age, geography, or practice setting. For example, being the “old guy in the room”, or as one woman reported, “When you are being put out there…everyone is going to be like this proves that she doesn’t know or she really doesn’t deserve to be here. And I just want to hide.” Lastly, participants and non-participants alike reported that they welcomed opportunities for technical or social vulnerability, stating its importance in the field of medicine. Participants in our study negotiated these vulnerabilities as they described deep-rooted beliefs that vulnerability was essential to the surgeon identity and a mechanism for technical and cultural improvement.
Conclusion:
Surgeons discussed technical and social vulnerability as pervasive among surgical coaching interactions. Participants also described the reasons why they believed both vulnerabilities are imperative in order to continue to improve professionally. Our results indicate the necessity of promoting a constructive, collaborative environment so that vulnerability can be nurtured in positive ways. This qualitative analysis illustrates the significance of vulnerability in surgical coaching interventions and continual professional development.
G. C. Linderman1, W. Lin2, M. Sanghvi1, R. D. Becher1, A. A. Maung1, B. Bhattacharya1, K. A. Davis1, K. M. Schuster1 1Yale University School Of Medicine,Surgery,New Haven, CT, USA 2Yale University,Statistics And Data Science,New Haven, CT, USA
Introduction: Laparoscopy is superior to open surgery for elective colectomy, but its role in emergency colectomy remains unclear. Previous studies were small and limited by confounding as surgeons may have selected lower risk patients for laparoscopy. We therefore studied the effect of attempting laparoscopy for emergency colectomies while adjusting for confounding using multiple techniques on large national databases.
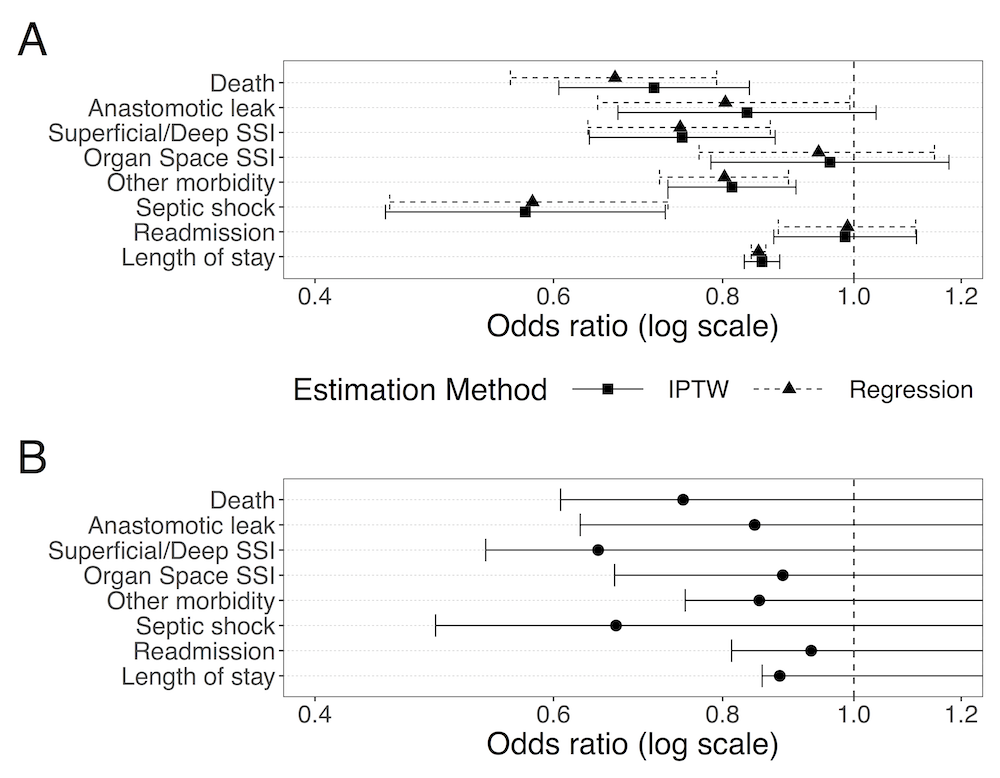
Methods: Using National Surgical Quality Improvement Program data, we identified emergency colectomy cases from 2014 to 2018. We first compared outcomes between patients who underwent laparoscopic versus open surgery, while adjusting for baseline variables using both propensity scores with inverse probability of treatment weighting (IPTW) and regression. Next, we performed a negative control exposure analysis using the apparent benefit of laparoscopy in cases converted to open. By assuming that the group which converted to open did not benefit from the attempt at laparoscopy, we used the observed benefit to bound the effect of unmeasured confounding on our estimates. Finally, we analyzed the effect of laparoscopy in the National Inpatient Sample (2009-2011) using an instrumental variable (IV) analysis, with an instrument based on proportion of cases done laparoscopically at each institution.
Results: Of 21,453 patients meeting criteria, 3,867 underwent laparoscopy, of which 1,375 converted to open. In both IPTW and regression analyses, attempting laparoscopy was associated with improved 30-day mortality, overall morbidity, anastomotic leak, surgical site infection (SSI), post-operative septic shock, and length of hospital stay (LOS) as compared to open surgery (Figure, Panel A). These effects do not appear to be driven by unmeasured confounding, as they were consistent with the lower bounds computed from the converted group (Figure, Panel B). IV analysis confirmed improved mortality and shorter LOS for laparoscopy.
Conclusions: Laparoscopic surgery for colorectal emergencies improves outcomes compared to open surgery. The benefit is observed even after adjusting for both measured and unmeasured confounding using multiple statistical approaches, thus suggesting a benefit not attributable to patient selection.