M. Oshi1, M. Okano1, A. L. Butash1, K. Takabe1 1Roswell Park Cancer institute,Department Of Surgical Oncology,Buffalo, NY, USA
Introduction: Despite the fact that the 5-year survival rate for breast cancer(BC) is outstanding compared to other cancers, there are 40,000 deaths annually due to the disease in the US. The vast majority of the mortality results from distant metastasis and therefore pre-clinical models are essential for development of proper and precise treatment for each patient. Patient-derived xenograft (PDX) maintains the features of the donor tumors such as intra-tumor heterogeneity. However, the establishment of an orthotopic metastatic model is still lacking due to procedural difficulty. We demonstrate our novel methods to develop an orthotopic brain metastasis patient-derived xenograft model (PDMOX) for BC brain metastasis.
Methods: PDMOX were created using metastatic brain tumors from BC patients and implanting them in the brain of NSG female mice aged 8-12m through a frontal bone burr hole into the right caudate putamen. Tumors of ~1mm3 were implanted in 2 different forms: single solid piece or mechanically minced tissue with medium.
Results:In the “manual push” method, a minced tumor mixed with 3µl medium is instilled at a depth of 4 mm by using a 23G needle, and a single solid piece of tumor is implanted by using forceps. In the “pipette tip” method, we utilized either a pipette for minced tissue, or a Hamilton syringe with a tip for solid tissue in order to inoculate tumor at the same depth. One hour post-surgical survival after implantation of minced tumor by “manual push” method was only 37.5% (3/8), whereas 100% (30/30) of the mice inoculated with the “pipette tip” method survived. The advantage of the “pipette tip” method was to minimize mechanical forces during inoculation into the brain by using a pipette or tip as a stopper. All tumors were well engrafted in surviving mice in both methods. With the “manual push” method, more tumors formed on the brain surface rather than within the brain parenchyma when compared to the “pipette tip” method. There was a large variation in tumor growth after “manual push”(Median 20±25.0, range: 12-24d. Tumor volume: median 5.6±21.0, range 2.8-48.7mm3). Although 2 out of 3 mice that underwent the “manual push” method had sudden death, all mice that underwent the “pipette tip” method lived until the tumor grew to 125-200mm3 without neurological symptoms. There was no difference in the time of engraftment and tumor growth rate between solid piece and minced tumor tissue using the “pipette tip” method. The success rate of passage for 2nd and 3rd generation was 100% (26/26).
Conclusion:Various surgical techniques used to generate PDMOX BC models showed major differences in the tumors and outcomes. These novel models are expected to become powerful tools for preclinical studies in metastatic BC.
N. G. Nicolson1, J. M. Healy1,2, R. Korah1, T. Carling1 1Yale University School Of Medicine,Yale Endocrine Neoplasia Laboratory, Department Of Surgery,New Haven, CT, USA 2Connecticut Children’s Medical Center,Hartford, CONNECTICUT, USA
Introduction: Next-generation sequencing has provided detailed genetic profiles of sporadic adrenocortical carcinoma (ACC). However, the genetic landscapes of ACC developing in patients with tumor predisposition syndromes are not well-characterized, as they are excluded from large-scale studies. Understanding the somatic genomic events complementing the background of germline syndromes is critical for designing personalized therapeutics for these unique patients, and may shed light on the molecular underpinnings of both sporadic and syndromic ACC.
Methods: ACC tissue and matched normal adrenal from a pediatric patient with clinically characterized Beckwith-Wiedemann syndrome were subjected to whole-exome sequencing (WES). Using multi-layered bioinformatics analysis, the WES data was compared to 21 sporadic ACCs which had also undergone WES. Single nucleotide variants (SNVs) of interest were subjected to damage prediction protocols to identify potential ACC drivers, and somatic copy number variations (CNVs) were also investigated.
Results: WES analysis revealed 8 relevant germline mutations in the index case, including a TP53 mutation previously associated with Li-Fraumeni syndrome. 25 potentially damaging somatic SNVs including multiple unique novel gene variants were identified. A damaging somatic mutation was identified in tumor suppressor CACNA2D3, a calcium channel gene in the same family as those previously reported to be mutated in some adrenocortical tumors. Although the mutation burden in the index case was similar to the sporadic ACC cohort, the total absence of CNVs was distinct from sporadic cases, all of which carried CNVs.
Conclusion: This study characterizes for the first time the genomic landscape of syndromic adrenocortical carcinoma of a patient with markers of both Beckwith-Wiedemann and Li-Fraumeni syndromes. The unique SNV and CNV profiles demonstrate that syndromic adrenocortical tumors may represent a genetically distinct entity from sporadic tumors.
S. Ruff1, R. Ayabe1, P. Malekzadeh1, M. Good1, M. Wach1, N. Nilubol1, E. Kebebew2, D. Patel1 1National Cancer Institute,Thoracic And Oncologic Surgery Branch,Bethesda, MD, USA 2Stanford University,Palo Alto, CA, USA
Introduction: There are no predictive clinical or laboratory tests that accurately distinguish between benign and malignant pheochromocytomas or paragangliomas (PPGLs). There is a link between dysregulated microRNAs and adverse prognosis in multiple malignancies. The overexpression of miR-210 has been found to be associated with the hypoxic state of the tumor microenvironment. The aim was to investigate if serum miR-210 levels could be used as a marker of malignancy in patients with PPGLs.
Methods: Patients with PPGLs (n=35) underwent an operation and had preoperative serum collected. Clinical demographics, genetic mutations, primary tumor size, postoperative biochemical response, and the development of recurrence or metastatic disease was prospectively collected. Total microRNA was extracted from the preoperative serum samples and miR-210 levels were measured by quantitative RT-PCR and normalized to miR-16. On univariable analysis primary tumor size, age, gender, race, body mass index, genetic mutation, miR-210 level, primary tumor site, and postoperative biochemical response from initial surgery was examined. Variables with a p-value <0.1 were included in a multivariable analysis.
Results: Of the 35 patients in our study, 10 underwent an operation for metastatic or recurrent disease and 25 did not develop recurrence or metastatic disease after their initial operation (median follow-up time of 65.7 months). Most patients had a primary pheochromocytoma (seven out of ten patients with metastasis/recurrence versus 17 out of 25 patients with benign pheochromocytomas). The most common mutation in the entire cohort was SDHB (n=10). There was a significantly lower serum miR-210 level in patients with metastasis/recurrence (2.3 versus 3.1, p=0.013) and larger primary tumor size in patients with a metastasis/recurrence (6.7 cm versus 4.1 cm, p=0.043) on univariable analysis. Age, gender, race, body mass index, genetic mutation, and postoperative biochemical response were not associated with recurrent or metastatic disease. Multivariable analysis did not show a significant difference for tumor size or miR-210 levels between these two groups.
Conclusion: Serum miR-210 expression levels were lower in patients with metastatic or recurrent disease at time of operation. Mir-210 expression may be a biomarker associated with metastatic or recurrent disease.
C. J. Park1, M. P. Shaughnessy1, R. A. Cowles1 1Yale University School Of Medicine,Pediatric Surgery,New Haven, CT, USA
Introduction: Animal models allow researchers to study the effects of pharmacological and surgical interventions on the gastrointestinal (GI) mucosa. The benefits of using mice in the laboratory include low cost, ease of maintenance, and the applicability of a wide range of molecular tools. While the typical laboratory mouse reaches sexual maturity at 6-8 weeks, maturation ensues and mice are considered mature adults at 12-24 weeks. Meanwhile, the ages and genders of mice used for experiments reported in the literature vary drastically. We hypothesized that there is age-, but no gender-related variation in intestinal morphometric parameters, necessitating thoughtful experimental design.
Methods: With IACUC approval, C56Bl/6J mice of varying ages (6, 12, 18, 24 weeks; n=4/group) were euthanized and the small intestine was isolated from the ligament of Treitz to the ileocecal valve. 2 cm segments of proximal, middle and distal small intestine were harvested and morphometric parameters assessed with microscopy. The distal segments were also stained for Ki67 to determine the crypt proliferation index (CPI). Secondary analysis comparing males and females (n=4/group) of matched ages was performed. Means were compared with Student’s t-test and variance of proportions was assessed with the Chi-squared test to a significance of p<0.05.
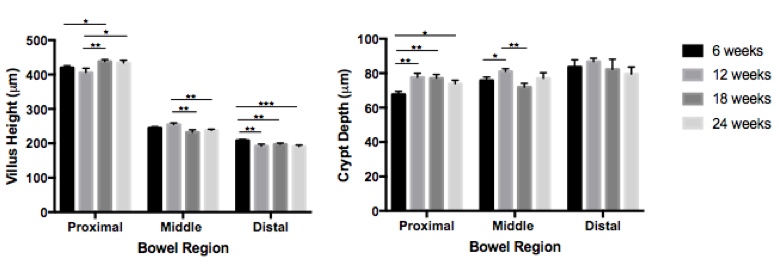
Results: There was variation in the measured morphometric parameters (Figure). In the proximal small intestine, 18-week old mice had taller villi compared to 6- and 12-week old mice and 24-week old mice had taller villi compared to 12-week old mice. In the middle segments, 12-week old mice had taller villi compared to 18- and 24-week old mice. In the distal segments, 6-week old mice had taller villi compared to all other age groups. When comparing crypt depth, 6-week old mice had shallower crypts compared to all other groups in the proximal region and 12-week old mice had deeper crypts compared to 6- and 18-week old mice in the middle segments. CPI was statistically lower only in 12-week old mice compared to all other age groups. When comparing age-matched males and females, there was no difference in villus height or crypt depth except for the middle small intestine where villus height was greater in females compared to males. There was no difference in CPI between genders.
Conclusion: Small, but statistically significant, differences in villus height, crypt depth and crypt proliferation are present in mice of different ages, while fewer differences exist between male and female mice of the same age. Investigators studying the GI mucosa should be aware of these differences and aim for consistent age-matching of experimental animals in order to avoid errors and allow direct comparison between studies.
A. Aggarwal1, Z. Yuan1, M. A. Nehs1 1Brigham And Women’s Hospital,Department Of Surgery,Boston, MA, USA
Introduction: Anaplastic thyroid cancer (ATC) is a fatal malignancy characterized by rapidly dividing tumor cells that demonstrate dependence on glycolysis for energy metabolism. We therefore sought to investigate the role of the glycolytic enzyme Hexokinase II (HK2) which is over expressed in many malignancies, including ATC. We hypothesized that a high glucose environment would promote HK2 gene expression and drive glycolytic energy capacity.
Methods: We cultured ATC cell lines (JL30 and 8505C) in high (25mM) or low (3mM) glucose concentrations for 96 hours. We analyzed HK2 expression by Fluorescent in-situ hybridization (FISH). We performed Seahorse XF Glycolytic stress tests to determine glycolytic reserve under each condition.
Results: We found higher and more variable HK2 expression in both ATC cell lines in the high glucose medium. JL30 had an average of 3.1 HK2 signals [Range 2-14] with high glucose environment versus 2.7 signals [Range 0-5] in the low glucose environment. Seahorse metabolic analysis revealed a glycolytic capacity of 245.8 mpH/min in the high glucose environment versus 55.9 mpH/min (p<0.001). 8505 had an average of 2.9 HK2 signals in the high glucose environment versus 2.7 in the low glucose environment. Seahorse metabolic analysis revealed a glycolytic capacity of 45.5 mpH/min for high glucose treatment versus 25.8 mpH in the low glucose treatment (p<0.01).
Conclusion: Here we demonstrate that a high glucose environment increases the expression of the glycolytic enzyme Hexokinase II and that this was associated with a higher metabolic glycolytic reserve. These studies are suggestive that the metabolism of anaplastic thyroid cancer cells is influenced by glucose concentrations. Future studies are needed to determine if hyperglycemia influences tumor biology in vivo.
M. K. Penny1, K. J. Basham2, S. Chukkapalli3, Y. Yu3, M. J. Hoenerhoff4, G. D. Hammer2,5, E. A. Newman3 1University Of Michigan,Doctoral Program In Cancer Biology,Ann Arbor, MI, USA 2University Of Michigan,Department Of Internal Medicine, Division Of Metabolism, Endocrinology And Diabetes,Ann Arbor, MI, USA 3University Of Michigan,Department Of Surgery, C.S. Mott Children’s And Women’s Hospital, Mott Solid Tumor Oncology Program,Ann Arbor, MI, USA 4University Of Michigan,Unit For Laboratory Animal Medicine,Ann Arbor, MI, USA 5University Of Michigan,Endocrine Oncology Program, Rogel Cancer Center,Ann Arbor, MI, USA
Introduction: Adrenocortical carcinoma (ACC) is a rare and often aggressive cancer, with a 5-year survival rate of 15% for those diagnosed with advanced disease. Improved models of this disease are needed for predictive preclinical testing in vivo. Subcutaneous, renal subcapsular, and splenic xenograft models of ACC do not mimic the endogenous tumor microenvironment and require injection of high cell quantity (≥ 2.5×106) for efficient tumor engraftment. The development of orthotopic ACC xenograft models has been significantly limited by the complexity and morbidity of open surgery. In this study, we introduce a novel model of orthotopic ACC xenografts utilizing ultrasound-guided percutaneous implantation directly into the adrenal gland tissue, using as few as 200,000 cells.
Methods: NCI-H295R and Y1 cells tagged with luciferase were percutaneously implanted into the adrenal of six to twelve-week old immunocompromised (NSG) mice. Mice were injected with 200,000 cells or 1,000,000 cells. Mice were monitored weekly by ultrasound and bioluminescent imaging and were euthanized and grossly dissected when tumors reached endpoint as defined by 150mm3 detected by ultrasound or 1011photons/sec/cm2/sr detected by bioluminescent imaging. The liver, lung, spleen, kidney, adrenal, and lymph nodes were examined histologically.
Results: At endpoint, 89% of mice injected with 200,000 Y1 cells, 80% of mice injected with 200,000 NCI-H295R cells, and 75% of mice injected with 1,000,000 NCI-H295R cells had locally-invasive primary tumor in the adrenal and periadrenal space. Y1 metastases were found in the liver and lung. Average time to endpoint was 5.2 weeks, 8.9 weeks, and 9.6 weeks respectively. Bioluminescent photon flux and maximum radiance were not linearly correlated with tumor size in vivo.
Conclusion: Minimally invasive orthotopic xenografts of ACC cells in NSG mice are an efficient and reliable method of developing biologically relevant tumors. Successful tumor growth and spontaneous metastasis following injection of as few as 200,000 cells per adrenal demonstrates the advantage of modeling of the tumor microenvironment, as compared to subcutaneous and more recently published renal and splenic xenograft models, as well as orthotopic xenografts by open surgery. This model is an important improvement over standard ACC models for preclinical testing of new therapeutics and investigation of tumor biology.
A. M. Mechler-Hickson1, M. Gowda1, S. L. Thibeault1 1University Of Wisconsin,Department Of Surgery, Division Of Otolaryngology – Head And Neck Surgery,Madison, WI, USA
Introduction:
The larynx is of vital importance in humans, contributing to breathing, swallowing, and phonation. It is also uniquely positioned at both the junction of the respiratory and GI tracts and the immunological boundary between the upper and lower respiratory systems. Due to their position, the vocal folds (VF), which are housed in the larynx, are exposed to a wide array of inhaled and ingested challenges. There is evidence that cells in the VF play a role in responding to these challenges: TLR1-6, TLR8 and TLR9 have been found in VF fibroblasts, the primary cell type of the lamina propria. Additionally, the majority of VF diseases are inflammatory in nature and have large economic and social consequences for patients. Despite the importance of the VF, and the inflammatory etiology of much of VF disease, mechanisms of host immunity in the larynx are ill-defined, and treatment of these inflammatory disorders is largely empiric. We worked to characterize toll-like receptors (TLR) 1-9, integral receptors in the innate immune system, in murine VF tissue, as mice are frequently used as a model species for humans, and in human VF epithelial cells.
Methods:
In order to localize TLR1-9 in murine VF tissue, excised larynges were coronally sectioned and processed for immunohistochemistry. Primary antibodies against TLR1-9 were used. An α-IgG secondary antibody was then applied, and diaminobenzidine was used as a staining agent. Staining was completed in biological and technical triplicate for each of the 9 antibodies with positive and negative controls.
Transcript levels of TLR1-9 were investigated in immortalized human VF epithelial cell lines. Cells were seeded into 6-well plates at a density of 1.5E5/mL and grown to near confluence. Cells were then treated with or without 5μg/mL LPS for 24 hours in wells intended for RT-PCR, and with or without 5μg/mL LPS for 8 hours in wells intended for ELISA analysis of IL-8, a downstream product of TLR activation. RT-PCR and ELISA were performed in biological and technical triplicate.
Results:
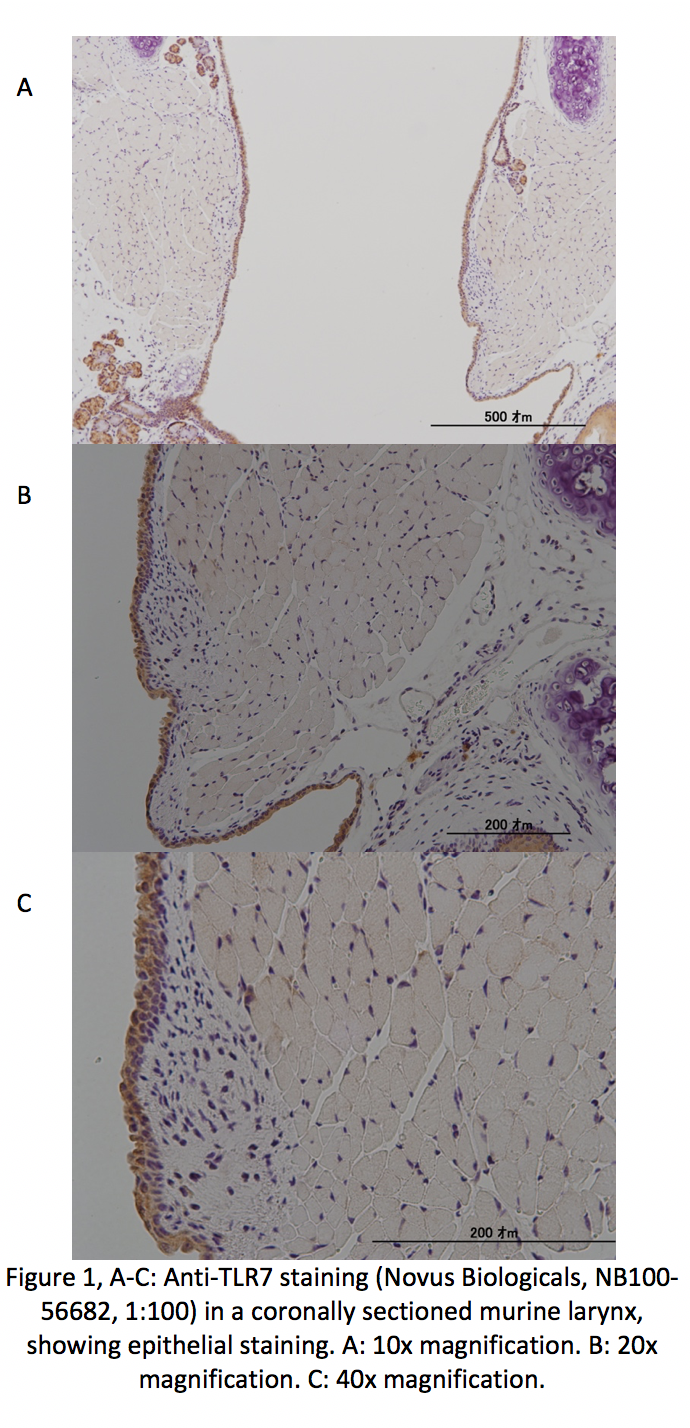
TLR1-9 were found in the epithelial cell layer of VF (Fig. 1). At the time of abstract submission results are ongoing, but we expect to find TLR1-9 in VF epithelial cells, with decreased expression for TLR6 -7. When treated with LPS we expect to find increased TLR expression and IL-8 levels, similarly to prior work in VF fibroblasts.
Conclusion:
This study localized TLR1-9 in VF epithelium. Exploring this, and how TLR expression and downstream cytokine activation differs between normal epithelial cells and those exposed to inflammatory stimuli, better elucidates how laryngeal epithelium responds to the variety of insults it is exposed to and may provide targets for treatment of airway disease.
L. A. DeFreest1, G. J. DeFreest-Rondeau1, L. Van De Water1, C. M. DiPersio1 1Albany Medical College,Department Of Surgery,Albany, NY, USA
Introduction: Integrins expressed in epidermal keratinocytes have important roles in cutaneous wound healing including paracrine stimulation of angiogenesis, regulation of proliferation and migration, and remodeling of extracellular matrix (ECM). Our laboratory has investigated roles of the laminin-binding integrin α3β1 in keratinocytes using both in vivo and in vitro models of wound healing. Our previous studies support a critical role for α3β1 in regulating the secretion of proteins into the extracellular milieu. Current data indicate a gene regulatory mechanism that involves crosstalk between α3β1 and estrogen responsive pathways in keratinocytes. Our long-term goal is to elucidate how α3β1 and estrogen coordinately regulate paracrine and autocrine signaling mechanisms that modulate the wound microenvironment to promote efficient wound healing.
Methods: For these studies, we have established a cell culture model consisting of mouse keratinocytes (MK cells), that are homozygous for a null mutation in the gene that encodes the α3 integrin subunit (i.e. lacking α3β1), or MK cells stably transfected with human α3 (i.e. MK α3+ cells, expressing α3β1). Conditioned media collected from these cells were compared by mass spectrometry (MS) to identify proteins secreted in an α3β1–dependent manner. Candidate proteins were then further evaluated by immunoblot to confirm and quantitate their expression/secretion. MK cells were also evaluated for the presence of estrogen receptor (ER) isoforms.
Results: 19 proteins identified by MS were upregulated more than 4-fold in medium from MK α3+ cells, compared with MK α3– cells. The secretion of lactoferrin, a protein whose production is controlled through an ER-dependent pathway, was up-regulated 18-fold in MK α3+ cells which was confirmed by immunoblot. Preliminary results indicate that MK cells express both ERα and ERβ. Furthermore, ERβ was increased in MK α3+ cells compared with MK α3- cells while ERα levels were similar in both cell lines. ERβ and ERα were increased in both MK lines by treatment with estrogen for 24 hours.
Conclusion: Proteomic analysis identified numerous secreted proteins that are upregulated by α3β1 indicating an important role for this integrin in controlling the keratinocyte secretome. Among these proteins, lactoferrin has been shown to modulate wound healing and has anti-microbial and immunomodulatory properties that make it an interesting candidate for further study. Lactoferrin is known to be induced by estrogen signaling, suggesting an unexplored intersection of pathways governed by integrins and ER. In particular, regulation of ERβ levels downstream of α3β1 may be a novel intracellular signaling pathway to regulate production of paracrine factors which in turn, modulate the wound microenvironment.
A. M. Kressel1,3,4, T. Tsaava1, E. H. Chang1, Q. Chang1, V. A. Pavlov1,2, S. S. Chavan1,2, K. J. Tracey1,2 1The Feinstein Institute for Medical Research,Center For Biomedical Science,Manhasset, NEW YORK, USA 2The Feinstein Institute for Medical Research,Center For Bioelectronic Medicine,Manhasset, NEW YORK, USA 3Northwell Health,Department Of Surgery,Manhasset, NEW YORK, USA 4The Elmezzi Graduate School of Molecular Medicine,Manhasset, NEW YORK, USA
Introduction: The inflammatory reflex is a well-defined neural circuit composed of afferent and efferent vagus nerve fibers that both senses peripheral cytokine levels and regulates splenic tumor necrosis factor (TNF) production to maintain homeostasis. Previous studies have demonstrated that efferent vagus nerve signals originate both in the dorsal motor nucleus of the vagus (DMV) and in the nucleus ambiguus in the brainstem. The efferent arc of the inflammatory reflex relays functional signals from the efferent vagus nerve fibers to the splenic nerve, which, after entering the splenic parenchyma, modulates the release of acetylcholine from a subset of T-cells and a subsequent attenuation of TNF production from resident macrophages. Although this pathway has been extensively studied by us and other groups, the existence of a functional synapse between the vagus and splenic nerves remains controversial. Using optogenetics and neural recordings, we selectively studied the role of DMV cholinergic neurons in inducing splenic nerve activation.
Methods: Using stereotactic guidance, a fiber optic cannula was inserted into the DMV of transgenic mice expressing channelrhodopsin under the choline acetyltransferase promoter (ChAT-ChR2-EYFP) and stimulation was applied (473nm laser, 20Hz, 25% duty cycle, 3 minutes, n=12/group). Splenic nerve activity was simultaneously recorded using a cuffed two-channel electrode. Next, to determine the contribution of the vagus nerve in transmitting signals generated in the DMV to the splenic nerve, we measured splenic nerve activity before and after chemically blocking the vagus nerve. Following recording of splenic nerve activity during DMV stimulation, 0.05% bupivacaine was applied to the vagus nerve to prevent further signal transduction, and splenic nerve activity was recorded again during DMV stimulation.
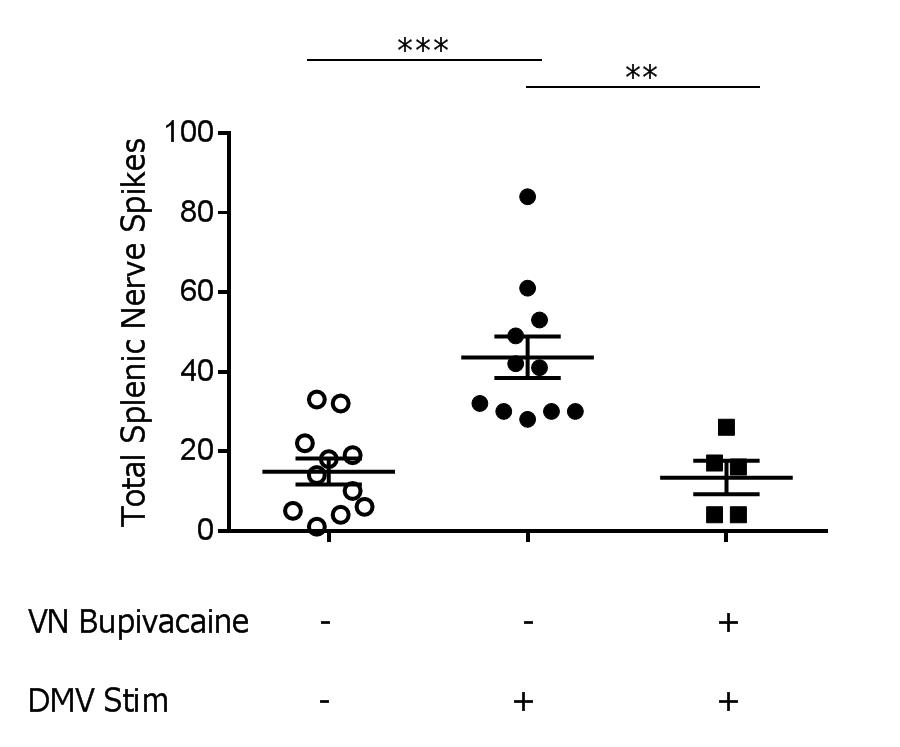
Results: Splenic nerve activity was significantly increased over baseline (p=0.0002) during optogenetic stimulation of the cholinergic fibers in the DMV (Figure 1). Administration of bupivacaine on the vagus nerve significantly attenuated splenic nerve activity during optogenetic stimulation of cholinergic fibers in the DMV (p=0.0091), demonstrating a functional synapse between the vagus and splenic nerves.
Conclusion: These studies reveal that cholinergic fibers originating in the DMV induce splenic nerve activation via the vagus nerve. Knowledge of the neural circuitry of the inflammatory reflex, coupled with our previous work on endotoxemia, will allow for therapeutic interventions for patients with inflammatory conditions.
M. Oshi1, M. Okano1, A. L. Butash1, K. Takabe1 1Roswell Park Cancer Institute,Department Of Surgical Oncology,Buffalo, NY, USA
Introduction: Although targeted therapies in primary breast cancer have significantly improved the survival rate in the last two decades, the challenge to improve the survival rate in patients with metastatic breast cancer still remains. Pre-clinical models play an important role in developing treatment strategies, but proper breast cancer metastasis models have not been established due to the difficulty and complication of the procedure. We developed the “thoracotomy” method in order to establish a breast cancer lung metastasis model, which is simple and resembles human lung metastasis.
Methods: All work was performed in female NSG or bulb/c mice of age 8-12 weeks. PDX of lung metastasis model was made from patient-derived breast tumors. PDX breast tumors that had been passaged 3 times in mammary fat pads or lung metastasis tumor generated using 4T1 cell line were used. Tumors were diced to ~1 mm3 pieces using a sharp blade.
Results: The right middle lobe was selected as an implantation site in order to allow the tumor to invade the lung and not the chest wall. In the “thoracotomy” method, the chest wall incision was made and tumor fragments were implanted using forceps and 8-0 nylon surgical suture. Another approach was to directly inject the minced tumor tissue 1mm below the lateral pleural surface of the middle lobe using a 23G needle. The incision was closed with a 6-0 surgical suture. An intrathoracic puncture was made with a 27G needle to withdraw the remaining air from the chest cavity. After the air had been withdrawn, a completely inflated lung could be seen through the thin chest wall. In the “non-thoracotomy” method, the minced tissue was injected into the mice lung through the chest wall with a 23G needle. One hour post-surgical survival rate was only 30% after “thoracotomy” method (non-fixing suture 9/30, fixing suture 8/30) due to open pneumothorax resulting from excessive wound tension and intercostal muscle cut through. All mice after “non-thoracotomy” method survived, but implantation in the chest wall was observed in 67% (4/6) of cases and the method achieved only 50% (3/6) of the accurate transplantation into the middle lung when performed preliminarily using the cell line. To increase the survival rate with the “thoracotomy” method, we limited the incision size <10 mm and compared the outcome with the original incision group. Limited incision “thoracotomy” could significantly increase one hour post-surgical survival to 97% (29/30) (<10 mm vs. ≥10 mm: t test P = 0.003).
Conclusion: By simple modifications of surgical techniques, we are able to establish an orthotopic lung metastasis mice model with almost zero operative mortality. Our orthotopic thoracotomy model has the potential to be a powerful tool for preclinical studies of breast cancer patients with lung metastases.
J. Tsuchida1, M. Nagahashi1, K. Moro1, M. Ikarashi1, Y. Koyama1, H. Ichikawa1, Y. Shimada1, J. Sakata1, T. Kobayashi1, H. Kameyama1, K. Takabe2,3, T. Wakai1 3University at Buffalo Jacobs School of Medicine and Biomedical Sciences, The State University of New York,Department Of Surgery,Buffalo, NEW YORK, USA 1Niigata University Graduate School of Medical and Dental Sciences,Division Of Digestive And General Surgery,Niigata, NIIGATA, Japan 2Roswell Park Comprehensive Cancer Center,Breast Surgery, Department Of Surgical Oncology,Buffalo, NEW YORK, USA
Introduction: A bioactive lipid mediator, sphingosine-1-phosphate (S1P) has emerged as a key regulatory molecule in cancer progression. S1P exerts its regulatory functions after it is secreted out of cancer and stromal cells and regulates various cellular functions, such as cell proliferation, migration, and angiogenesis. We previously demonstrated that S1P is a crucial mediator of breast cancer-induced angiogenesis and lymphangiogenesis and promotes metastasis to the lymph nodes and to the lung (Cancer Res 2012, Cancer Res 2018). We have also reported that high S1P levels in the tumor are associated with lymph node metastasis in breast cancer patients (J Surg Res 2016), however, the role of S1P in plasma has not been fully investigated in breast cancer patients. In this study, we studied the levels of sphingolipids including S1P in plasma from breast cancer patients to reveal their clinical significance.
Methods: A retrospective analysis was conducted on 88 breast cancer patients, who received curative surgery in our institute. Among them, 18 patients received neoadjuvant chemotherapy. The plasma from the patients were obtained immediately prior to the operation. Sphingolipids, including sphingosine, dihydro-sphingosine, S1P and dihydro-S1P, were determined by liquid chromatography-electrospray ionization-tandem mass spectrometry (LC-ESI-MS/MS). The levels of sphingolipids were analyzed with patients’ clinical demographics, and statistical analysis was performed with the Mann- Whitney U-test.
Results: The levels of sphingolipids including sphingosine, dihydro-sphingosine, S1P, dihydro-S1P were detected successfully in the plasma from all the breast cancer patients. Levels of S1P in patients with neoadjuvant chemotherapy (N=18, median 1144, range 657–1555 pmol/ml) was significantly higher than that in patients without neoadjuvant chemotherapy (N=70, median 797, range 448–1827 pmol/ml) (P<0.0001). Levels of sphingosine in patients with neoadjuvant chemotherapy (median 25, range 17–61 pmol/ml) was significantly higher than that in patients without neoadjuvant chemotherapy (median 20, range 9–76 pmol/ml) (P=0.0143). Among the 70 patients without neoadjuvant chemotherapy, S1P levels in patients with pathologically proven lymph node metastasis (N=17, median 884, range 698–1275 pmol/ml) was significantly higher than that in patients without lymph node metastasis (N=53, median 758, range 448–1827 pmol/ml) (P=0.0091). Sphingosine levels in the patients with pathologically proven lymph node metastasis (median 22, range 12–76 pmol/ml) was also significantly higher than that in the patients without lymph node metastasis (median 19, range 9–63 pmol/ml) (P=0.0340).
Conclusion: Our results suggest that S1P in plasma plays an important role during the process of lymph node metastasis in breast cancer patients. It also implicates a possibility of plasma S1P as a biomarker for lymph node metastasis.
L. S. CHATURVEDI1,2,3, D. VYAS1,3 1SAN JOAQUIN GENERAL HOSPITAL,SURGERY,FRENCH CAMP, CALIFORNIA, USA 2CALIFORNIA NORTHSTATE UNIVERSITY,PHARMACEUTICAL SCIENCES AND BIOMEDICAL SCIENCES,ELK GROVE, CALIFORNIA, USA 3CALIFORNIA NORTHSTATE UNIVERSITY,COLLEGE OF MEDICINE,ELK GROVE, CALIFORNIA, USA
Introduction:
It is estimated that one million cases of breast cancer are diagnosed annually worldwide. Of these, approximately 12-20% are of the triple-negative breast cancer (TNBC) that do not express receptors for estrogen (ER), progesterone (PR) or human epidermal growth factor receptor 2 (HER2). TNBC is typically treated with a combination of other therapies such as chemotherapy, radiation, and surgery. Therefore, there is urgent need of new therapy for TNBC patients. Carfilzomib (CFZ) is a selective irreversible second-generation proteasome inhibitor being used for the treatment of relapsed and refractory multiple myeloma as the anticancer therapy. We have previously reported antiproliferative and apoptotic effects of CFZ alone or in combination with Doxorubicin (DOX) on human TNBC-MDA-MB-231 cancer cells. Overexpression of cyclin-dependent kinase inhibitor CDKN1A/p21Waf1/Cip1 and an elevated phosphorylation of stress-activated c-Jun NH2-terminal kinase has been reported in basal-types and TNBCs with poor prognosis. However, the role of CFZ in the regulation of CDKN1A/p21Waf1/Cip1 protein expression and c-Jun NH2-terminal kinase activation has not been determined in human TNBC-MDA-MB-231 cancer cells. Herein, we investigated the role of CFZ in the modulation of CDKN1A/p21Waf1/Cip1 and c-Jun NH2-terminal kinase activation in human TNBC-MDA-MB-231 breast cancer cells.
Methods:
Human TNBC-MDA-MB-231 cell-line, a model for TNBC was treated by various concentrations of CFZ alone, in a combination with DOX or vehicle control dimethyl sulfoxide (DMSO). Cell Counting Kit-8 (CCK-8) using WST-8 (2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2, 4-disulfophenyl)-2H-tetrazolium, monosodium salt) was used to detect cell viability. Annexin V-FITC apoptosis detection kit and flow cytometry were used to analyze the cell cycle. Western blot was used to detect the expression of cyclin-dependent kinase inhibitor CDKN1A/p21Waf1/Cip1 protein and phosphospecific c-Jun NH2-terminal kinase antibodies
Results:
We confirmed the antiproliferative and apoptotic effects of CFZ alone or in combination with DOX using CCK-8 viability and Annexin V-FITC apoptosis detection Kit respectively. The immunoblot analysis revealed that CFZ alone significantly inhibited CDKN1A/p21Waf1/Cip1 in a dose-dependent manner and as well as in a combination with DOX in comparison to vehicle control DMSO. Furthermore, CFZ alone and in a combination with DOX also significantly inhibited the phosphorylation of stress-activated c-Jun NH2-terminal kinase in human TNBC- MDA-MB-231 cancer cells.
Conclusion:
Our data suggest that irreversible proteasome inhibitor CFZ alone and in combination with DOX may induce apoptosis and inhibit proliferation by modulating CDKN1A/p21Waf1/Cip1 protein expression and phosphorylation of stress-activated c-Jun NH2-terminal kinase in human MDA-MB-231 breast cancer cells. Further investigation will encourage the potential use of CFZ alone and in the combination of DOX against tumors harboring drug-resistant phenotypes.
A. -. Maiti1, M. Okano1, I. Okano1, T. Kawaguchi1, K. Takabe1, N. Hait1, A. Maiti1 1Roswell Park Cancer Institute,Surgical Oncology,Buffalo, NY, USA
Introduction: ~~Breast cancer most often recurs and metastasizes to the distal organs that had their primary tumors surgically excised. Functional analysis of organ-specific metastasis genes suggested that there might be two different categories of metastasis genes. One group of genes has the role in tumor growth and survival besides their metastasis function while another group of genes only have a specific role in facilitating adaptation of tumor cells at distant sites and do not have a clear role in promoting primary tumor growth.In this study, we assessed the specific cancer-related gene expression changes occurring with metastatic breast cancer recurrence to distant organs comparing with non-metastatic breast cancer.
Methods: ~~RNA-sequencing was done using two cell lines 4T1-luc2 that can only metastasize to lung and 4T1.2-luc2 metastasized to bone and lung suggested that at least 50 genes which are statistically highly expressed in 4T1.2-luc2 compared to 4T1-luc2 cells. Out of which only a few genes well established for cancer metastasis biology include ANGPTL77, SERPINE2, TSPAN11, ESM1, LCN2 which expressed over 8 fold in 4T1.2-luc2 compared to 4T1-luc2 cells. A metastatic syngeneic mouse model was developed and metastatic growth to distant organs was monitored using IVIS and MRI imaging after the primary tumor was surgically excised from mice.
Results:~~Our animal model tumor molecular analysis data revealed that LCN2 (7 fold) and CD133 (above 20 fold) over-expressed in the spine and bone compared to the primary or lung met lesions formed by 4T1.2-luc2. Conversely, LCN2 and CD133 genes are downregulated in breast cancer lung metastasis tissues, while CD151, EPHA2, and TWIST1 genes highly overexpressed in lung metastatic lesion compared to primary, bone or spine. Further, the RNA-seq data of patient samples explained that LCN2, CD133 expressions are significantly higher (8 out of 10 patients) in advanced breast cancer bone metastases compared with matching non-metastatic breast cancer.
Conclusion:~~Our data suggested that LCN2, CD133 would be a prognostic marker for breast cancer spinal bone metastasis, while CD151, TWIST1, EPHA2 would be a prognostic marker for lung metastasis and organ-specific relapse.
J. Leckenby1,3, M. Chacon1, A. Grobbelaar3, J. Lichtman2 1University Of Rochester,Plastic Surgery,Rochester, NY, USA 2Harvard School Of Medicine,Molecular And Cell Biology,Cambridge, MA, USA 3Royal Free Hospital NHS Foundation Trust,Plastic Surgery,London, LONDON, United Kingdom
Introduction:
Peripheral nerve assessment has traditionally been studied through histological and immunological staining techniques in a limited cross-sectional modality. The introduction of transgenic species, such as YFP-H mice, has greatly increased our ability to observe axonal regeneration and subjectively comment on behavior. However, detailed analysis is still difficult to assess with either of these methods and understanding cellular interactions is almost impossible. A new application of serial section electron microscopy (SSEM) is presented to overcome these limitations and open the application of this technique to other areas of pathology.
Methods:
Direct nerve repairs (DNR) were performed on the posterior auricular nerve of transgenic YFP-H mice. Six weeks post-operatively the nerves were imaged using confocal fluorescent microscopy then excised and embedded in resin. Resin blocks were sequentially sectioned at 100nm and sections were serially imaged with an electron microscope (Magellan 400L, FEI) utilizing the following parameters: 100nm pixel size, 200ns Dwell Time, 8k x 8k pixel field of view with 7.0kV and 26nA. Images were aligned and auto-segmented to allow for 3D rendering using 3D Studio Max (Autodesk).
Results:
Basic morphometry and axonal counts were fully automated. Using full 3D reconstructions, the relationships between the axons, the Nodes of Ranvier, and Schwann cells could be fully appreciated (See Fig. 1). The quality of regeneration could be examined throughout the entire dataset providing a comprehensive analysis. The interactions of individual axons with their surrounding environment could be visualized and explored in a virtual three dimensional space.
Conclusion:
SSEM allows the detailed pathway of the regenerating axon to be visualized in a 3D virtual space in comparison to isolated individual traditional histological techniques. Fully automated histo-morphometry can now give accurate axonal counts, provide information regarding the quality of nerve regeneration and reveal the cell-to-cell interaction at a super-resolution scale. It is possible to fully visualize and ‘fly-through’ the nerve to help understand the behavior of a regenerating axon within its environment. Having established this technique it provides future opportunities to evaluate the affect different treatment modalities have on the neuro-regenerative potential and help us understand the impact different surgical techniques have when treating nerve injuries. Further investigations are being carried out to determine whether SSEM can be used to analyze cellular interactions in disease processes such as infection or tumor formation.
E. Katsuta1, L. Yan2, K. Takabe1 1Roswell Park Cancer Institute,Breast Surgery, Department Of Surgical Oncology,Buffalo, NY, USA 2Roswell Park Cancer Institute,Department Of Biostatistics And Bioinformatics,Buffalo, NY, USA
Introduction: The use of clinical targeted DNA-sequencing to detect genomic alterations, including mutation and copy number alterations has become a routine in clinical practice for targeted therapy. However, the interpretation of the results in each gene is still understudied. MYC is one of the essential oncogenes and it plays a crucial role in regulation of the cell cycle and proliferation in various types of cancers including Triple-Negative Breast Cancer (TNBC). It is known that MYC amplification and high expression are associated with TNBC; however, the difference between a MYC amplified vs high expressing tumors in TNBC is not fully elucidated.
Methods: Clinical and genomic data, including mRNA and Genomic Identification of Significant Targets in Cancer (GISTIC), were obtained from The Cancer Genome Atlas (TCGA) through cBioportal. MYC amplification was defined based upon copy-number GISTIC; GISTIC 2 was defined as tumor with amplification, remaining GISTIC -1, 0 and 1 were defined as tumor without amplification.
Results: Among 1080 patients with DNA copy-number data, 229 tumors (21.2%) showed MYC amplification, which was the most common copy-number alteration in the whole TCGA breast cancer cohort. Although MYC mRNA expression level was higher in MYC amplified tumors compared to non-amplified tumors (p<0.001), the mRNA expression levels were highly overlapped between amplified and non-amplified tumors. MYC amplification (p=0.112) as well as high expression (p=0.390) were not associated with overall survival (OS) in the whole cohort. 156 patients (15.5%) were classified as TNBC based on ER, PgR, immunohistochemistry status and HER2 immunohistochemistry and FISH method. In agreement with previous reports, there was greater proportion of TNBC subtypes in MYC amplified tumors (p<0.001), as well as in MYC high expressing tumors (p<0.001). Thus, we focused on TNBCs. There was no significant difference in MYC mRNA expression level between MYC amplified and non-amplified tumors in the TNBCs (p=0.074). Interestingly, none of the clinicopathological demographics were associated with either MYC amplification or high expression. However, high expression of MYC was significantly associated with worse OS (5-year OS rates: 61.6% vs 78.3%, p=0.026), whereas MYC amplification was not associated with OS (5-year OS rates: 75.7% vs 70.9%, p=0.515) in TNBC. Gene Set Enrichment Analysis (GSEA) demonstrated that MYC target gene sets (v1; p<0.001, v2; p<0.001) as well as cell cycle related gene sets, including E2F targets (p=0.013) and G2/M check point (p=0.018) gene sets, and WNT/beta-catenin gene set (p=0.017) were significantly enriched in MYC high expressing tumors, whereas out of 50 hall mark gene sets, none of them were enriched in MYC amplified tumors.
Conclusion: TNBCs with MYC higher expression, but not amplification, has worse prognosis in TNBC. It is important to consider that amplification is not always equal to high expression in some genes.
H. M. Poushay1, M. Asaoka1, K. Takabe1 1Roswell Park Cancer Institute,Department Of Surgical Oncology,Buffalo, NY, USA
Introduction:
Immunotherapy has revolutionized treatment of many cancers, although its role in breast cancer treatment has yet to be well-established. Immune checkpoint inhibitors (ICIs) have demonstrated significant therapeutic responses across a variety of cancers, and may prolong overall survival in tumors with high mutation loads. One such ICI is the programmed death-1/programmed death ligand-1 (PD-1/PD-L1) inhibitor, which targets the PD-1/PD-L1 pathway, resulting in restoration of the immune system’s anti-tumor response. To investigate the potential role of ICIs in the treatment of breast cancer, we sought to compare hypermutated breast cancers to non-hypermutated breast cancers. We hypothesized that hypermutated breast cancers would have increased heterogeneity, leading to an increased presence of tumor-infiltrating lymphocytes (TILs), which has been shown to portend favorable response to immunotherapy. We also hypothesized that the hypermutated group would have higher cytolytic activity and PD-1/PD-L1 activity; the latter has also been suggested as a predictor of cancer response to PD-1/PD-L1 inhibitor therapy.
Methods:
Genomic and clinical data of 1065 breast cancer patients were obtained from The Cancer Genome Atlas (TCGA) and the Pan-Cancer Atlas.
We defined hypermutated tumors as those with non-silent mutation rate greater than 3.0.
Cytolytic activity (CYT), T cell receptor (TCR) diversity, and tumor infiltrating immune cell composition were calculated by CIBERSORT. Categorical variables were compared by Fisher's exact test (p<0.05 considered significant).
Results:
Of the 1065 patients, 114 (10.7%) were identified as having hypermutated tumors. The incidence of hypermutation was more frequent in older patients (age ≥50 vs. <50; 12.1% vs. 7.4%; p=0.03; odds ratio [OR]=1.7), in ER-negative compared to ER-positive tumors (18.6% vs. 8.8%; p <0.01; OR=2.4), but was not associated with cancer stage (p=0.485) or HER2 receptor status (p=0.079). Intra-tumor heterogeneity was higher in hypermutated tumors (p=0.014). Activated CD4 T-cells (p<0.001), macrophage M1 (p<0.001) and gamma delta T-cells (p<0.001) were higher in hypermutated tumors, whereas immune restraining regulatory T cells were lower (p=0.004). Reflecting the infiltration of immune activating cells, gene expression associated with TILs (p<0.001) and TCR diversity (p<0.001) were higher in the hypermutated group. Expression of immune checkpoint molecules PD-1 (p<0.001) and PD-L1 (p<0.001) were increased in the hypermutated tumor group. Finally, immune cytolytic activity was higher in the hypermutated group (p<0.001).
Conclusion:
The results support our hypothesis that hypermutation breast cancer tumors have increased heterogeneity and cytolytic activity, and increased PD-1/PD-L1 activity. Given these findings, further study is warranted to investigate the potential role of ICIs in breast cancer treatment in selected breast cancer patients.
R. R. Sheldon1, M. Loughren1, C. Marenco1, K. Morte1, W. S. Do1, D. T. Lammers1, J. B. Weiss1, D. M. Forte1, V. Y. Sohn1, M. J. Martin1, M. J. Eckert1, R. O. Burney1, S. T. Marko1, J. Bingham1 1Madigan Army Medical Center,Tacoma, WA, USA
Introduction: Microdermal implants are an increasingly popular form of body jewelry in which the device is surgically implanted beneath the skin and held in place by scar tissue that forms around the dermal anchor. The potential for electrical conduction burn at the site of metal jewelry left in situ during electrosurgery prompted surgical societies to recommend routine removal. However, routine removal of microdermal implants can be complicated, requiring destruction of the device or local excision. Given the paucity of evidence regarding electrosurgical burn risk associated with microdermal implants, we assessed in vivo thermal effect and tissue damage at implants during electrocautery use.
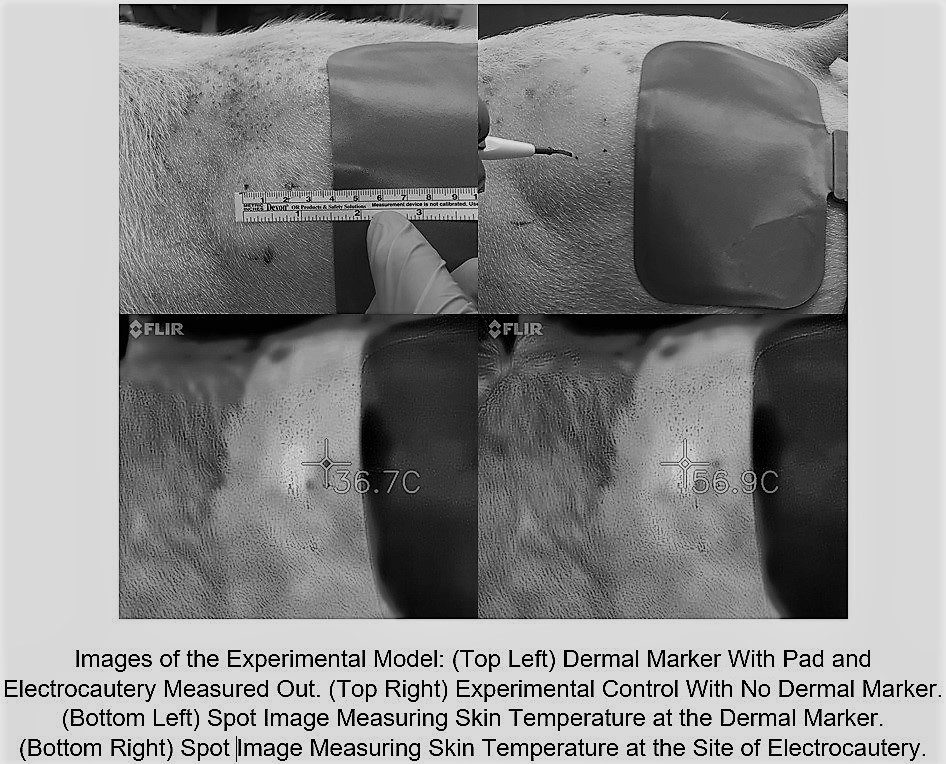
Methods: Stainless steel microdermal anchors (3 mm) were surgically implanted into 4 adult swine. Implants were placed into the bilateral flank and one of two randomized hindquarters. After 4 days to allow for initial healing and scar tissue formation, the bilateral flank implants were evaluated and excised for histologic examination. A Bovie electrocautery grounding pad was placed 2 cm caudal to the microdermal implant. Continuous electrocautery (coagulation/ 30 watts) for 30 seconds was applied to the skin at a point 2 cm cranial to the dermal implant. Surface skin temperature was recorded during electocautery using thermal imaging. Tissue damage was assessed by both gross examination and histologic evaluation of tissue immediately surrounding the excised microdermal implant. The same procedure was then performed to the contralateral non-implanted side as a sham control.
Results: Electrocoagulation for 30 continuous seconds raised the skin temperature around the electrocautery tip 49.9° F to an average of 148.7° F. Skin around the dermal implant only rose 3.24° F to an average of 101.7° F whereas the control skin without the dermal implant rose 3.65° F to 102.3° F (P=0.627). Skin temperatures at the dermal implant at 5 second intervals throughout the coagulation interval showed no statistical difference to those taken from the sham side at any time point. Histologic review of excised tissue samples showed no evidence of thermal injury.
Conclusion: Dermal implants appear to have no effect on skin temperature change during the use of electrocautery even when they are in close proximity to both the grounding pad and site of electocautery use. This suggests that microdermal implants may be safe and aggressive steps to ensure their removal before surgery are unnecessary.
T. Takeshita1, M. Okano1, E. Katsuta1, X. Peng2, L. Yan2, K. Takabe1 1Roswell Park Cancer Institute,Surgical Oncology,Buffalo, NY, USA 2Roswell Park Cancer Institute,Biostatistics & Bioinformatics,Buffalo, NY, USA
Introduction: The androgen receptor (AR) is one of the members of the steroid nuclear receptor family, which includes estrogen receptor (ER) and progesterone receptor (PR). AR is expressed in 50–90 % of breast cancers. The role of AR in breast cancer is mechanistically complex and remains controversial. Some in vitro data have shown that androgen and AR have a role in proliferation of normal and malignant breast tissues. High AR expression also demonstrated resistance to tamoxifen and aromatase inhibitors in both in vitro and in vivo systems. The possible mechanism of this resistance was that breast cancer tumor cells could be changed from ER-dependent to AR-dependent. Further, it has been demonstrated that AR supports estradiol-mediated ER activity in ER/AR both positive breast cancer cells. In this study, we investigated the association of AR mRNA and protein expression and patient survival using gene and protein expression data of the publically available large cohort.
Methods: Clinical, gene and protein expression data were obtained from The Cancer Genome Atlas (TCGA) and METABRIC through cBioPortal. Disease free survival (DFS), overall survival (OS) , gene set enrichment analysis (GSEA) and CIBERSORT analysis were conducted comparing high and low AR expression groups, which were defined as lower quartile based upon previous publications.
Results: AR high and low expression group were 817 and 272 patients in TCGA whole cohort and 1068 and 356 patients in METABRIC whole cohort, respectively. AR expression was significantly higher in ER positive tumors compared to ER negative tumors (p<0.001) in both cohorts. The high expression AR group showed significantly worse OS in ER positive patients in TCGA cohort (p=0.007). In METABRIC cohort, AR high group showed significantly worse OS in Luminal B patients (p=0.007). No significant difference in survival was observed by AR protein expression in TCGA cohort. To explore the mechanism of these results, GSEA was conducted. As expected, it was demonstrated that androgen response related gene set was significantly enriched with AR mRNA high expression (Normalized enrichment score; NES=1.75, p=0.003). Protein secretion related gene set (NES=1.76, p=0.01) and estrogen response related gene set (NES=1.67, p=0.02) were also significantly enriched with high AR. On the other hand, DNA repair related gene sets was significantly enriched in AR low expressed tumors in ER positive tumors (NES=-1.75, p=0.01). In CIBERSORT analysis, AR high tumors were negatively associated with immune-eliminating cells, such as CD8 T-cells, Gamma-Delta T-cells and memory B-cells (p>0.01).
Conclusion: High expression of AR showed worse progress in ER positive breast cancer. High AR expression tumor was enriched estrogen response related gene expression that might associate with worse OS in ER positive patients.
J. Whitt1, A. Aune2, N. E. Avalon3, B. J. Baker3, H. Chen1, R. Jaskula-Sztul1 1University Of Alabama at Birmingham,Surgery,Birmingham, Alabama, USA 2Auburn University,Pharmacy,Auburn, ALABAMA, USA 3University of South Florida,Chemistry,Tampa, FLORIDA, USA
Introduction:
Neuroendocrine tumors (NETs) may arise from neuroendocrine cells located throughout the body, but usually occur in the gastrointestinal tract, lungs, or thyroid. Clinical trials have demonstrated a response rate of only 20% for single agent chemotherapy, leaving surgery as the only cure. However, the disease has usually metastasized by the time of diagnosis and widespread metastases make complete surgical resection impossible. Thus, there is a need to identify new therapeutics that will reduce NET development and progression, while improving patient quality of life. Recent experiments from our group and others have demonstrated a tumor suppressor function of the Notch pathway in NETs. Natural compounds belonging to the trichothecene and cytochalasin families have previously demonstrated anti-proliferative effects against various cancer types. We investigated the potential therapeutic effect of these compounds via the reduction of cell proliferation and activation of the Notch pathway in NETs.
Methods:
Fourteen compounds isolated from Myrothecium verrucaria and other fungal species were received from the Chemistry Department at the University of South Florida. NET cell lines BON, H727, TT, and MZ cell lines were treated with several concentrations of the compounds. For comparison, the non-cancerous WI-38 cell line was treated with the same concentrations of the compounds. Cell viability and cell cycle was assessed using MTT and flow cytometry, respectively. RNA was isolated from treated and untreated cells and RT-PCR was used to analyze changes in gene expression.
Results:
Two compounds significantly reduced cell viability in a dose-dependent manner. These compounds, Cytochalasin D and Roridin E, exhibited low micromolar IC50 values in BON, H727, MZ, and TT cell lines. Both Roridin E and Cytochalasin D increased Notch1 expression within 24 hrs, which was followed by a decrease in cell viability. Roridin E also decreased the expression of ASCL1, a transcription factor important for the development of NETs.
Conclusion:
Two compounds were identified as transcriptional activators of Notch1 signaling. This is the first time that a specific effect on Notch signaling has been identified for these compounds. Our findings support the development of these compound classes for the treatment and palliation of patients with NETs, which can significantly enhance the therapeutic outcome of NE cancer therapy while minimizing undesirable side-effects.
K. K. Rossfeld1, D. Huk2, S. E. Justiniano2, M. Saji2, L. A. Shirley1, M. D. Ringel2, L. S. Kirschner2, J. E. Phay1 1Ohio State University,Surgical Oncology,Columbus, OH, USA 2Ohio State University,Endocrinology, Diabetes, And Metabolism,Columbus, OH, USA
Introduction: Patients with locally aggressive and metastatic thyroid cancer have limited treatment options. Preclinical animal modeling is an important step in developing new therapies for these patients. Existing thyroid cancer mouse models promote tumor growth either through orthotopic injection of cancer cells or via genetic alterations. Unfortunately, tumor growth in the thyroid gland in these models often causes local compression or invasion requiring early euthanasia. We sought to determine whether surgical resection of the thyroid without the demise of the animal was feasible.
Methods: Mice with thyroid-specific deletion of Pten develop follicular adenomas, and mice with thyroid-specific deletion of Prkar1a develop follicular thyroid cancer (FTC). Mice with combination Pten and Prkar1a thyroid deletion develop vascular FTC; half develop pulmonary metastases. We have recently described a medullary thyroid cancer (MTC) orthotopic xenograft model. Five mice were selected for surgical resection, one with Pten deletion, one with Prkar1a deletion, and one with an MTC orthotopic xenograft, along with two mice with double Prkar1a/Pten deletion.
Results: Three of the five mice survived surgical resection of their tumors. The Pten knockout mouse survived 6 months postoperatively. The Prkar1a knockout mouse had a local recurrence and required euthanasia one month following surgical resection. The mouse with the MTC orthotopic xenograft survived resection but also developed local recurrence and required sacrifice after three months. Both Prkar1a/Pten knockout mice suffered perioperative mortality.?
Conclusion: Survival after surgical resection of a thyroid neoplasm in the murine model was demonstrated in three of four models. The mortalities seen in Prkar1a/Pten knockout mice were secondary to difficulty with vascular control as well as hyperthyroidism. Interestingly, local recurrence of the resected tumor was observed in two cancer models, which is often a clinical challenge in patients with thyroid cancer. These models may allow for further investigation of molecular mechanisms underlying local recurrence, novel surgical techniques, such as image-guided surgery, and novel chemotherapies for metastatic disease. ?
G. Urrutia1, J. Toro-Zapata1, A. Salmonson1, G. Lomberk1 1Medical College Of Wisconsin,Research/Surgery,Milwaukee, WI, USA
Introduction: Pancreatic ductal adenocarcinoma (PDAC) presents a significant health burden as the third leading cause of cancer-related deaths in the United States. Despite significant efforts to develop better therapeutics to treat PDAC, the 5-year survival rate for patients has improved only marginally. Thus, there remains an urgent need to further understand the molecular mechanisms underlying PDAC development to identify innovative therapeutic targets. Our laboratory is focused on utilizing epigenetic inhibitors for this purpose.
Methods: IncuCyte® Live-Cell Analysis and clonogenic assays were utilized to monitor the in vitro effect of drug treatments (LY2606368 and BRD4770) on L3.6 and primary PDAC cell lines from human patients. Cellular effects were observed by immunofluorescence of phosphorylated H2A.X and FACS analyses, while molecular effects were assessed by western blot for various cell cycle and apoptosis markers. Subcutaneous xenografts were implanted and subsequently treated with vehicle, individual drugs, or in combination to evaluate in vivo tumor growth, and immunohistochemistry was performed on the harvested tumor tissue.
Results: Here, we sought to combine targeting of Checkpoint kinase 1 (Chk1), a key regulator of cell cycle transition in the DNA damage response pathway, and G9a, an epigenetic regulator of histone H3 lysine 9 methylation (H3K9me), which is necessary for reforming chromatin during DNA replication. Using live cell imaging, we found that the growth of PDAC cells, both L3.6 and primary cell lines from PDAC patients, is reduced by the combined inhibition of Chk1 (LY2606368) and G9a (BRD4770), achieving a synergistic effect. This result was recapitulated by clonogenic assays. To determine the underlying mechanism, we evaluated the extent of DNA damage as measured by H2AX phosphorylation and found the combination of LY2606368 and BRD4770 induces activation of the ATR-Chk1 axis, but fails at the Chk1 checkpoint, leading to replication stress and DNA damage. FACS analysis of cells treated with the combination further supported this observation, which demonstrated a significant increase of cells in S-phase, as well as a substantial sub-G1 fraction, indicating cell death. Moreover, cell death coincided with increased levels of cleaved caspase 3, as confirmed by fluorescent detection and western blot. Interestingly, pan-caspase inhibition, using Z-VAD-FMK, did not rescue the effect, indicating that the main mechanism involved in this process is not caspase-dependent. In vivo treatment of subcutaneous pancreatic cancer xenografts demonstrated that combined targeting of these pathways reduces tumor growth, which involves a reduction in proliferation observed by Ki67 staining along with an increase in overall DNA damage.
Conclusion: In summary, our results demonstrate that targeting the epigenetic regulator G9a in combination with inhibition of the DNA damage response checkpoint offers a novel therapeutic approach for pancreatic cancer through triggering DNA replication catastrophe.
P. Murthy1, M. S. Zenati1, A. Al-Abbas1, C. Rieser1, M. T. Lotze1, A. Zureikat1, B. A. Boone1,2 1University of Pittsburgh,Surgery,Pittsburgh, PA, USA 2West Virginia University,Surgery,Morgantown, WV, USA
Introduction: Pancreatic ductal adenocarcinoma (PDAC) is an aggressive malignancy characterized by inflammation and a tolerogenic immune response. Neoadjuvant therapy is becoming increasingly utilized, particularly for borderline resectable disease. Due to the fibrotic nature of PDAC, radiographic responses after neoadjuvant therapy do not correlate with treatment response. As a result, biomarkers of response are needed. The systemic immune-inflammatory index (SII) has recently emerged as a predictor of survival in patients with PDAC when assessed at diagnosis. Our aim was to determine the prognostic significance of SII following neoadjuvant therapy for PDAC.
Methods: We retrospectively analyzed all PDAC patients treated with neoadjuvant therapy followed by pancreatic resection at the University of Pittsburgh Medical Center between 2007 and 2017. Pre- and post-treatment neutrophil, platelet, and lymphocyte values were collected to calculate SII ([Neutrophils x Platelets] / Lymphocytes). Pretreatment, post-treatment and change in SII following neoadjuvant therapy were evaluated for association with clinical outcomes, including margin negative resection, lymph node status, and survival. Cox regression and Kaplan-meier were used to evaluate survival. Harrell’s C and Somers’ D were used to find the optimal cutoff for SII in predicting survival.
Results: A total of 419 patients with PDAC treated with neoadjuvant therapy were studied. The median pre-treatment SII was 563 (IQR 367, 988) and the median post-treatment SII was 582 (275, 1140). There was a median decrease in SII following neoadjuvant treatment of 11% (-53%, 80%). There was a statistically significant association between change in Ca 19-9 with treatment and change in SII (p=0.029). Post-treatment SII was significantly lower in patients who had a Ca 19-9 response of >50% with treatment (Median 502 vs. 648, p=0.047). There was no significant correlation with pre-treatment SII and outcomes. Neither absolute change or percent change of SII following treatment were predictive of clinical outcomes. Post-treatment SII did not predict R0 resection, lymph node status or recurrence-free survival, but was independently associated with overall survival (OS) as a continuous variable (p=0.018). Our analysis demonstrated that a post-treatment SII of 900 was the optimal cutoff for predicting survival. Patients with a post-treatment SII > 900 had lower overall survival (Figure 1, 31.9 vs 26.1 months median OS, p = 0.0509).
Conclusion: Neoadjuvant therapy impacts the prognostic value of the system immune-inflammatory index (SII) as pre-treatment SII did not predict outcomes in this cohort. Post-treatment SII may be a useful prognostic marker in PDAC patients treated with neoadjuvant therapy, particularly for patients in whom Ca 19-9 is not a marker.
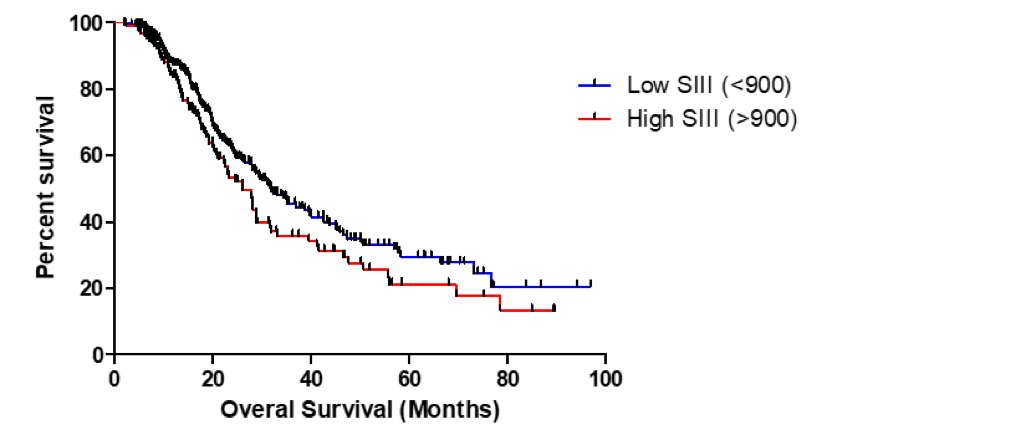
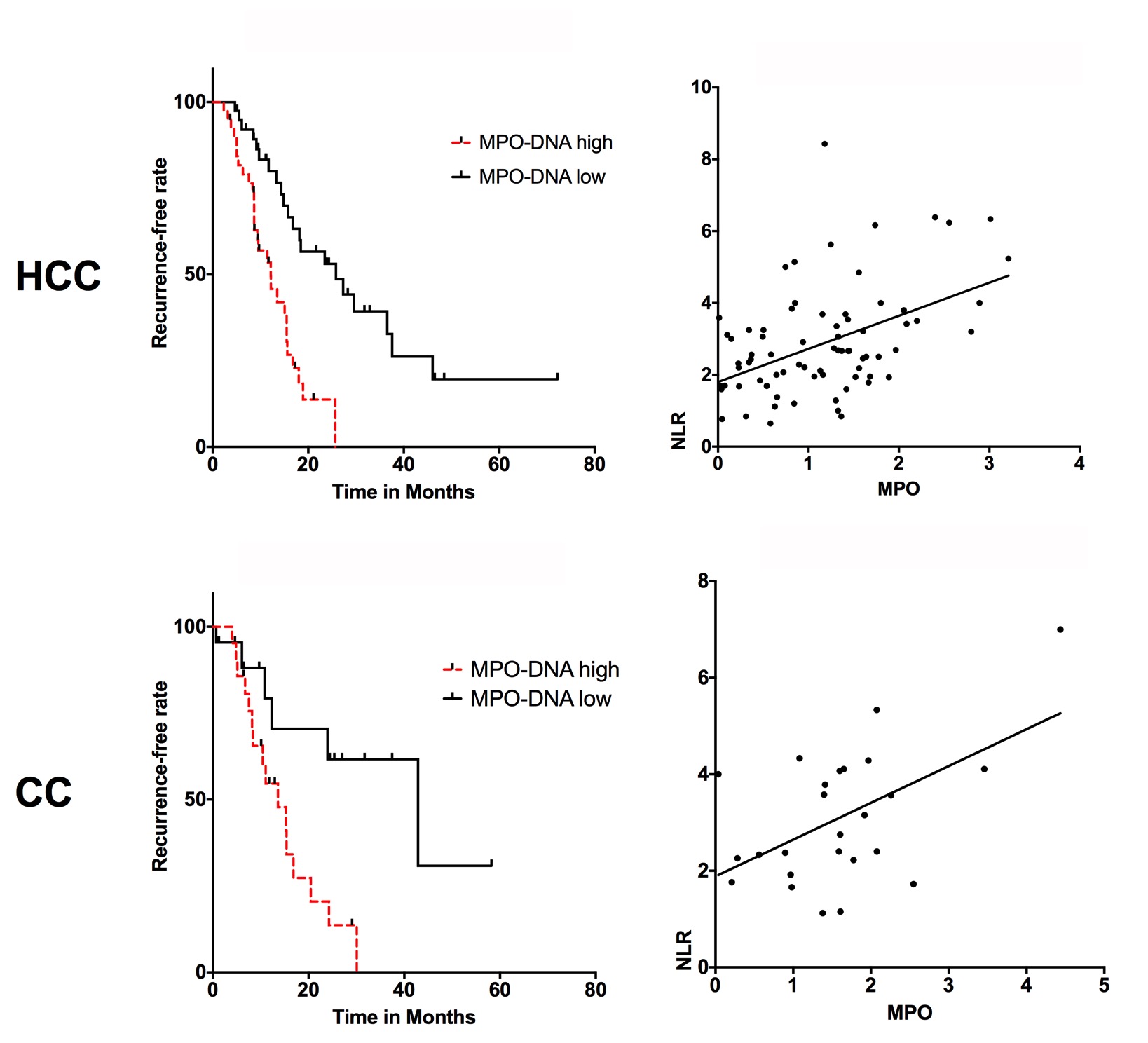
C. T. Kaltenmeier1, S. Tohme1, H. Yazdani1, D. Van Der Windt1, H. Huang1, A. Tsung1 1University Of Pittsburgh,Surgery,Pittsburgh, PA, USA
Introduction: A systemic inflammatory state is widely considered as a preoperative risk factor for outcomes in solid organ malignancies. Neutrophils have been known to play an important role during early and chronic inflammation. Neutrophil to lymphocyte ratio (NLR) is a well-recognized sensitive measure of inflammation and high NLR levels have been linked to risk of cancer recurrence. After activation, neutrophils release their DNA into the extracellular space, referred to as neutrophil extracellular traps (NETs). This is a defense mechanism first described to trap and kill bacteria and other pathogens, however has recently been identified in the pathogenesis of inflammatory and malignant diseases. We have recently shown that neutrophil extracellular traps (NETs) play a critical rule and can promote the development and progression of liver metastases after surgical stress in mice.
The current study uses a specific neutrophil marker – neutrophil myeloperoxidase (MPO-DNA) as a measure of NET formation. MPO is released into the extracellular space during neutrophil degranulation. We hypothesize that NETs can be utilized as a biomarker determining outcomes after resection of hepatic malignancies.
Methods:
We selected 103 consecutive patients with Hepatocellular carcinoma (HCC) or Cholangiocarcinoma (CC) who underwent surgery at our institution. Preoperative serum levels were routinely collected for tissue banking. Neutrophil number and MPO-DNA levels were measured pre-surgery. We then performed log rank analysis of recurrence-free survival in patients with high vs low MPO-DNA/NLR. In addition, Pearson correlation of pre-operative MPO-DNA and NLR was performed.
Results:
Pre-therapy MPO-DNA levels are strongly associated with recurrence-free survival in patients undergoing surgery for HCC or CC. Patients with higher pre-therapy MPO-DNA were more likely to have a shorter disease-free survival compared to those with low levels. (HCC: HR: 2.909, 95% CI: 1.607 to 5.264, p<0.0001, CC: HR: 3.221, 95% CI: 1.335 to 7.773 p<0.0093). Median survival for HCC patients with high vs low MPO-DNA was significant with 12.6 vs 25.8 months. Similar results were obtained in CC patient, high vs low MPO DNA was significant with 13.6 vs 42.9 months. In addition, there is a significant correlation between pre-therapy NLR and MPO-DNA for both HCC and CC (HCC: p<0.0001, R2 = 0.22, CC: p<0.0065, R2 = 0.28) (Figure 1).
Conclusion:
Neutrophils are an important marker for hepatic cancers after resection. The current study focuses on pre-therapy levels of MPO-DNA as a prognostic marker of recurrence free survival following surgery. This study showed that high pre-therapy NET levels are indicative of poor outcomes in patients undergoing surgery for HCC and CC.
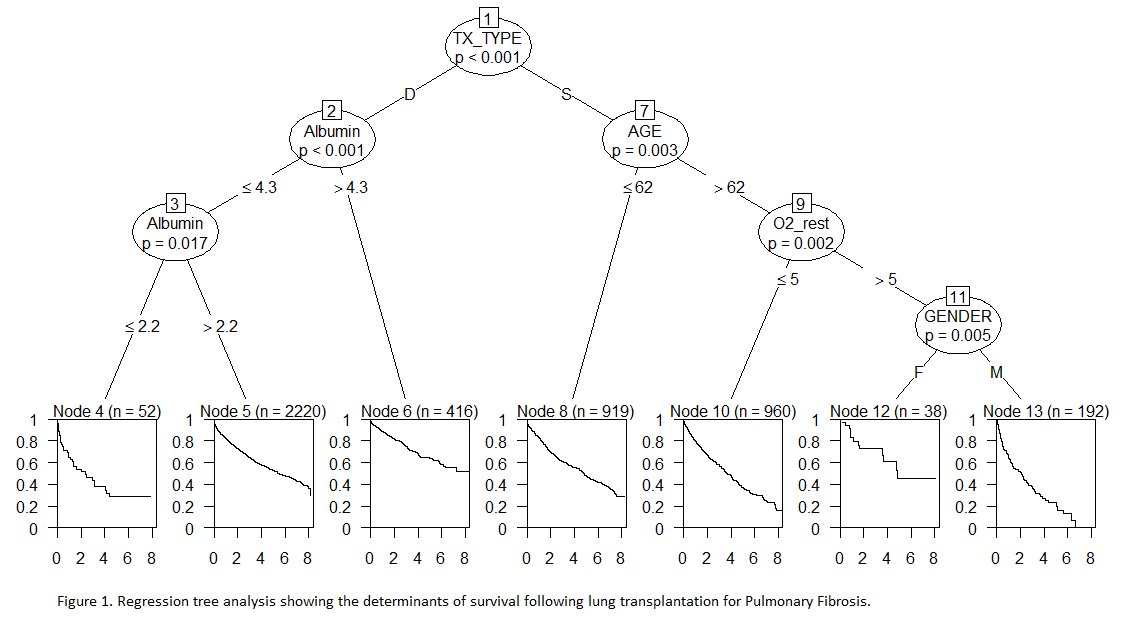
A. M. White1, A. L. Halpern1, P. D. Koltz1, F. L. Grover1, C. D. Scott1, J. D. Mitchell1, M. R. Zamora1, R. A. Meguid1,2, L. J. Helmkamp2, D. A. Fullerton1, M. J. Weyant1 1University Of Colorado Denver,Cardiothoracic,Aurora, CO, USA 2The Adult and Child Consortium for Health Outcomes Research and Delivery Science,University Of Colorado,Aurora, CO, USA
Introduction: In this review, we evaluate patients with Interstitial Lung Disease (ILD) or Interstitial Pulmonary Fibrosis (IPF) as a single consortium of Pulmonary Fibrosis patients requiring transplantation. Our objective was to evaluate the effect of risk factors on survival in patients with Pulmonary Fibrosis who underwent lung transplantation using the United Network of Organ Sharing (UNOS) national database.
Methods: This is a retrospective cohort study using propensity matched multivariable regression and survival tree analysis models to examine multivariable associations between preoperative variables and mortality using data from 2005 to 2012 from the UNOS national database. Patients with a diagnosis of ILD, IPF, or subset of either who received a single or bilateral lung transplant were included in the study. Survival tree analysis was used to find important predictors of survival from a set of candidates including transplant type, serum albumin level, age, gender, pulmonary artery pressure, oxygen requirement at rest, BMI, six minute walk test, FEV1, and FVC percent of expected.
Results: The national database included 4,797 patients meeting criteria, with 2,109 undergoing SLTx and 2,688 undergoing BLTx. Overall, in a propensity matched analysis, BLTx had improved survival (HR 0.76, 95% CI 0.65, 0.87). In regression tree analysis showing the factors influencing survival, the first determinant is type of transplant, with bilateral lung transplant conferring improved survival (p-value <0.001). The second determinant of improved survival was high serum albumin pre-transplant, defined as > 4.3 g/dL (p-value < 0.001), with low serum albumin pre-transplant (defined as ≤ 2.2 g/dL) predictive of significantly decreased survival. Age ≤ 62 was also predictive of increased survival (p-value 0.003) as was lower oxygen requirement at rest and female gender.
Conclusion: In patients with Pulmonary Fibrosis receiving lung transplantation, the type of lung transplant received and serum albumin are important determinants of survival.
R. Hamura1,2, Y. Shirai1,2, Y. Shimada2, N. Saito1,2, T. Horiuchi1,2, H. Sugano1,2, N. Takada1,2, T. Taniai1,2, Y. Kanegae3, T. Ohashi2, K. Yanaga1 1The Jikei University School of Medicine,Department Of Surgery,Minato-ku, TOKYO, Japan 2Research Center for Medical Science, The Jikei University School of Medicine,Division Of Gene Therapy,Minato-ku, TOKYO, Japan 3Research Center for Medical Science, The Jikei University School of Medicine,Core Research Facilities Of Basic Science (Molecular Genetics),Minato-ku, TOKYO, Japan
Introduction: Autophagy plays an important role in metabolism of anticancer agents and chemoresistance. Suppression of autophagy is expected to be a new strategy for cancer. Because autophagy depends on hydrolysis by lysosome enzymes, we hypothesized that down-regulation of lysosomal enzymes may induce autophagy dysfunction and enhance chemosensitization. In this study, we knocked down the gene of an lysosomal enzyme acid-αglucosidase (GAA), to evaluate the anti-tumor effects on pancreatic cancer.
Methods: The autophagy levels, apoptosis signals, cell viabilities and the enzyme activities of GAA were assessed using gemcitabine-resistance pancreatic cancer cell lines (PANC-1 and MIA PaCa-2) by Western blot analysis, cell viability MTT assay and GAA enzyme activity assay. These experiments were assessed by GAA knock down with siRNA.
Results: In both cell lines, the expression levels of LC3-II and LAMP2 protein were increased and p62 was deceased by gemcitabine. The expression levels of GAA protein were elevated by gemcitabine. Also, the enzyme activities of GAA were elevated by treatment of gemcitabine in a dose dependent manner (PANC1; gemcitabine (1μM) 120±8.0 % of control, p=0.034, MIA PaCa-2; gemcitabine (0.5μM); 157±7.3 % of control, p<0.01). Knockdown of GAA protein enhanced gemcitabine-induced expression levels of apoptotic signals (Cleaved caspase-3, -8, -9 and cleaved PARP), which were superior to the effects by an autophagy inhibitor, bafilomycin A1. Moreover, antiproliferative effects were increased by suppression of GAA as compared to gemcitabine mono therapy (MIA PaCa-2; gemcitabine vs. siGAA+gemcitabine, 110 ± 6.00 vs. 89.7± 9.69% of control, p<0.01).
Conclusion:Down-regulation of GAA enzyme activity induces autophagy dysfunction and enhances anti-tumor effect of gemcitabine on pancreatic cancer.
