P. Murthy1, M. S. Zenati1, A. Al-Abbas1, C. Rieser1, M. T. Lotze1, A. Zureikat1, B. A. Boone1,2 1University of Pittsburgh,Surgery,Pittsburgh, PA, USA 2West Virginia University,Surgery,Morgantown, WV, USA
Introduction: Pancreatic ductal adenocarcinoma (PDAC) is an aggressive malignancy characterized by inflammation and a tolerogenic immune response. Neoadjuvant therapy is becoming increasingly utilized, particularly for borderline resectable disease. Due to the fibrotic nature of PDAC, radiographic responses after neoadjuvant therapy do not correlate with treatment response. As a result, biomarkers of response are needed. The systemic immune-inflammatory index (SII) has recently emerged as a predictor of survival in patients with PDAC when assessed at diagnosis. Our aim was to determine the prognostic significance of SII following neoadjuvant therapy for PDAC.
Methods: We retrospectively analyzed all PDAC patients treated with neoadjuvant therapy followed by pancreatic resection at the University of Pittsburgh Medical Center between 2007 and 2017. Pre- and post-treatment neutrophil, platelet, and lymphocyte values were collected to calculate SII ([Neutrophils x Platelets] / Lymphocytes). Pretreatment, post-treatment and change in SII following neoadjuvant therapy were evaluated for association with clinical outcomes, including margin negative resection, lymph node status, and survival. Cox regression and Kaplan-meier were used to evaluate survival. Harrell’s C and Somers’ D were used to find the optimal cutoff for SII in predicting survival.
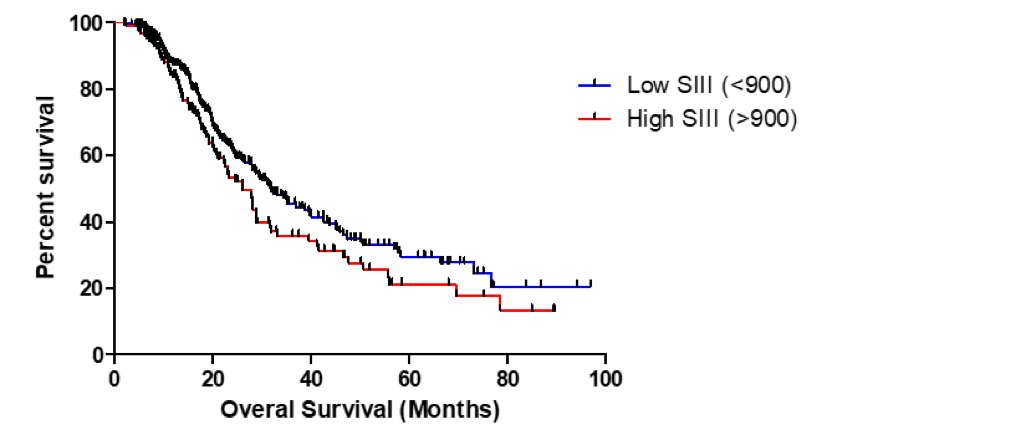
Results: A total of 419 patients with PDAC treated with neoadjuvant therapy were studied. The median pre-treatment SII was 563 (IQR 367, 988) and the median post-treatment SII was 582 (275, 1140). There was a median decrease in SII following neoadjuvant treatment of 11% (-53%, 80%). There was a statistically significant association between change in Ca 19-9 with treatment and change in SII (p=0.029). Post-treatment SII was significantly lower in patients who had a Ca 19-9 response of >50% with treatment (Median 502 vs. 648, p=0.047). There was no significant correlation with pre-treatment SII and outcomes. Neither absolute change or percent change of SII following treatment were predictive of clinical outcomes. Post-treatment SII did not predict R0 resection, lymph node status or recurrence-free survival, but was independently associated with overall survival (OS) as a continuous variable (p=0.018). Our analysis demonstrated that a post-treatment SII of 900 was the optimal cutoff for predicting survival. Patients with a post-treatment SII > 900 had lower overall survival (Figure 1, 31.9 vs 26.1 months median OS, p = 0.0509).
Conclusion: Neoadjuvant therapy impacts the prognostic value of the system immune-inflammatory index (SII) as pre-treatment SII did not predict outcomes in this cohort. Post-treatment SII may be a useful prognostic marker in PDAC patients treated with neoadjuvant therapy, particularly for patients in whom Ca 19-9 is not a marker.