A. L. Franklin1, T. Iordanskaia1, M. Barberio1, D. Pillai1, M. Hubal1, E. P. Nadler1 1Children’s National Health System,General Surgery,Washington, DC, USA
Introduction:
The pathogenesis of non-alcoholic fatty liver disease (NAFLD) is associated with obesity and insulin resistance. Hispanics have the highest prevalence compared to non-Hispanic Whites and Blacks, with Black patients having the lowest prevalence. Differences in the incidence of NAFLD among race/ethnicity may be related to visceral adipose tissue (VAT), as Blacks have less VAT than Whites and Hispanics. The exact mechanisms behind the ethnic and racial discrepancies are unclear. Exosomes are cell-derived vesicles that contain messenger RNA, microRNA, and proteins, that are potential mediators of systemic disease via the delivery of genetic material to distant organs. We believe that adipocyte-derived exosomes may be the direct mechanistic link between obesity and NAFLD, and exosomal differences may explain the ethnic and racial disparities. We have previously shown that adipocyte exosomes from mixed race/ethnicity donors lead to a dysregulation of gene expression of transforming growth factor beta-1 (TGFΒ-1) mediators in cultured hepatocytes. We sought to determine if ethnic differences among adipocyte exosomes exist.
Methods:
Institutional Review Board approval and patient or parental/guardian consent was obtained. HepG2 cells were grown to 60-70% confluence and then co-cultured with TGFΒ-1 for 48 hours. VAT exosomes from one Black (BMI 51) and one Hispanic (BMI 46) female patient were applied to HepG2 cells in culture with and without TGFΒ-1 exposure. PAI-1 protein expression was analyzed with ELISA, and compared via t-test. Experiments were performed in duplicate and repeated once for each donor.
Results:
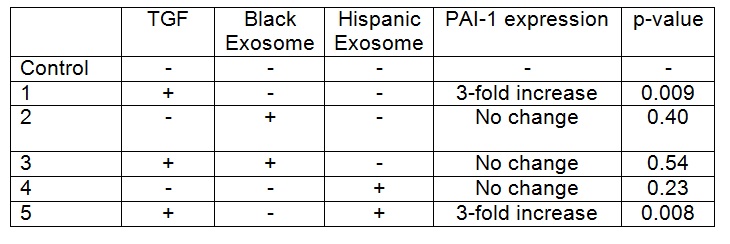
TGFB-1 exposure to HepG2 cells increased PAI-1 expression when compared to control cells (p= <0.05). Addition of exosomes from the Black patient decreased PAI-1 expression in HepG2 cells exposed to TGFB-1 (p=0.034), with expression levels that were similar to baseline (Table). In contrast, there was no difference in PAI-1 expression in HepG2 cells exposed to TGFB-1 and exosomes from the Hispanic patient when compared to cells co-cultured with TGFB-1 alone. Exosomes from neither donor altered PAI-1 expression in the absence of TGFB (p= >0.05).
Conclusion:
HepG2 cells co-cultured with TGFΒ-1 had an increase in PAI-1 protein expression. VAT exosomes from a Black patient inhibited TGFB-1 induced production of PAI-1 in hepatocytes. However, exosomes from a Hispanic patient did not inhibit this TGFB-1 induced upregulation of PAI-1. Racial differences in exosomal content and function likely exist, and may be the mechanism behind the known ethnic and racial differences in NAFLD prevalence.