S. G. Patel1, L. Li1, A. Maas1, R. Ghukasyan1, J. Williams1, P. Toste1, A. Nguyen1, I. Elliott1, N. Wu1, T. Donahue1 1University Of California Los Angeles,Surgery,Los Angeles, CA, USA
Introduction:
We have demonstrated that pancreatic cancer (PDAC) tumor associated fibroblasts (TAFs) support pancreatic cell growth and invasion from a pro-inflammatory, DNA damage (DDR) mediated pathway. This pathway referred to as the senescence associated secretory phenotype (SASP) requires both stress kinases and Nfkb signaling for maximum signal production after stimulus. Within the tumor microenvironment, genotoxic chemotherapies can stimulate the stromal cells to produce factors that promote tumor cell fitness. Contrary to the prevailing model, we have identified that JNK isoforms are cell-type specific and that both JNK1 and JNK3 can transduce this inflammatory pathway in the tumor microenvironment. We additionally have found that not all of the SASP is regulated by Nfkb, but rather a key mediator, IL-1 alpha, can be expressed in its absence.
Methods:
Immortalized pancreatic cancer tumor associated fibroblasts (Logsden Lab) as well as Hela cells were used to create both gene knockouts as well as stable knockdowns for JNK1, JNK3 and p65 (Nfkb signaling). SASP induction was verified after a DNA damage response using RTPCR. Small molecules specific to JNK isoforms were used to verify genetically manipulated cell lines in WT cells.
Results:
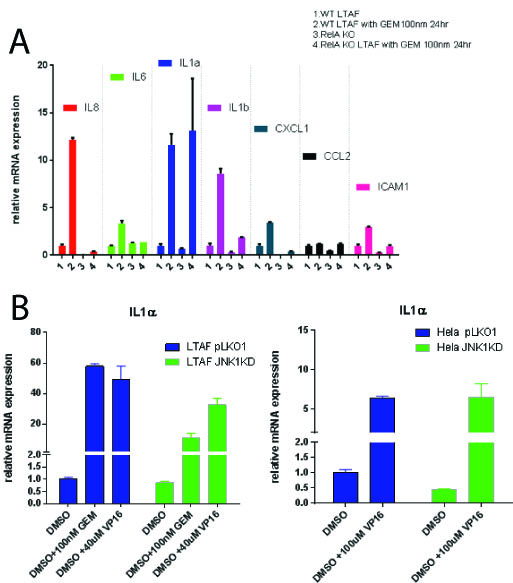
Gemcitabine treatment (100 nm) of PDAC TAFs induced a pro-inflammatory gene expression program including statistically significant (p < 0.05) upregulation of a number of cytokines: including IL-6, IL-8, IL-1 alpha and beta, CXCL1, CCL2 and ICAM1. Homozygous knockout TAFs for p65 (TAF p65 -/-) stops the induction of these all genes tested (p<0.05) except IL-1 alpha (p = 1) [Figure 1A]. Nfkb (p65) is required for upregulation of IL-1 alpha in the presence of recombinant (10 ug/mL) TNF-alpha or IL-1 alpha (p <0.05). We found that TAFs do not express JNK3, but rather JNK1 and 2. We identified using a combination of shRNAs to JNK1 and JNK3 as well as small molecule inhibitors to JNK1/2 that TAFs require JNK1 for upregulation of IL-1 alpha after a DDR (p<0.05) [Fig 1B]. This implies that in the tumor microenvironment, the SASP is propagated by specific stress kinases expressed.
Conclusion:
Previous studies have attributed the SASP to be critically dependent on Nfkb signaling as well as amplified in culture with JNK3. We identify that some elements of the SASP are not dependent on Nfkb signaling. Furthermore, we find that JNK3 is not a ubiquitous stress kinase in this response, but rather JNK isoforms are cell-type restricted in expression. These findings highlight the complexity of the pro-inflammatory signaling within the tumor microenvironment and suggest a more careful analysis of cell and tissue specific gene expression is needed for developing optimal treatment strategies.