J. Carr1, S. King1, C. Dekaney1 1University Of North Carolina At Chapel Hill,Chapel Hill, NC, USA
Introduction: Enteritis is a significant side effect of chemotherapy, and is a potential dose-limiting complication. Pharmacotherapy can diminish symptoms, but there are no effective therapies to prevent or treat the intestinal epithelial injury associated with enteritis, most likely because the mechanisms guiding epithelial damage and repair are unknown. Our preliminary studies suggest that enteric bacteria play a critical role in precipitating mucosal damage after treatment with doxorubicin (Dox), a common chemotherapeutic drug. Following Dox, mice treated with broad-spectrum antibiotics (ABRX) do not show the small intestine crypt loss, increased crypt depth, or alterations in proliferation demonstrated in wild type mice (WT). Bacteria and bacterial products signal via distinct toll like receptors (TLRs), while MyD88 is a key adapter molecule utilized by nearly all TLRs to activate downstream signaling pathways. ‘Knocking out’ MyD88 in the intestinal epithelial cell (IEC) specifically eliminates IEC bacterial signaling, and therefore allows an evaluation of the role of IEC bacterial signaling in mediating the bacterial contribution to enteritis. In the current study, we utilize this knock out model to determine whether elimination of bacterial IEC signaling is responsible for protection from Dox-induced enteritis.
Methods: At 10 weeks of age, control or IEC-specific MyD88 KO mice (VillinCre/MyD88fl/fl) were treated with a single intraperitoneal dose of Dox (20 mg/kg) and euthanized five days following treatment. The jejunum was fixed and stained with H&E for histologic analysis.
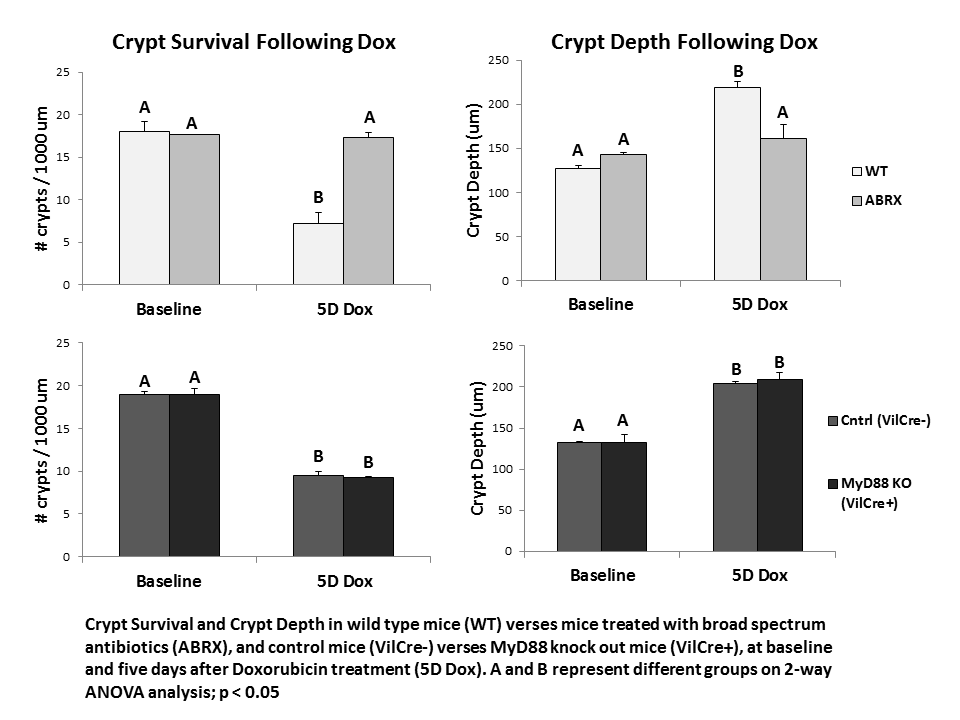
Results: While ABRX mice demonstrate significantly less weight loss than WT mice five days after Dox, MyD88 KO mice demonstrated weight loss equivalent to WT mice (WT 19.5% ± 2.4; ABRX 5.1% ± 4.6; MyD88 KO 17.5% ± 3.0). ABRX mice demonstrate significantly greater crypt survival than WT mice five days after Dox, and do not demonstrate increased crypt depth typical of WT mice in their hyper-proliferative state after Dox, while MyD88 KO mice demonstrate a phenotype comparable to WT mice (graph).
Conclusions: In response to insult with the chemotherapeutic Dox, MyD88 KO mice demonstrate intestinal damage followed by hyper-proliferation similar to WT mice. This suggests that the protection from damage demonstrated in ABRX mice is not due to elimination of epithelial bacterial signaling. Future studies will investigate other potential signaling pathways (e.g. penetration of a compromised epithelial barrier) to elucidate how depletion of bacteria protects from Dox-induced damage. This will allow determination of a prospective molecular target for prevention of chemotherapy-induced enteritis.