B. W. Tillman1,4, Y. Chun3, T. Richards1, T. Maul3, N. L. Liang1, A. Tevar2 1University Of Pittsburgh,Division Of Vascular Surgery, Department Of Surgery,Pittsburgh, PA, USA 2University Of Pittsburgh,Starzl Transplant Institute, Department Of Surgery,Pittsburgh, PA, USA 3University Of Pittsburgh,Department Of Bioengineering,Pittsburgh, PA, USA 4University Of Pittsburgh,McGowan Institute For Regenerative Medicine,Pittsburgh, PA, USA
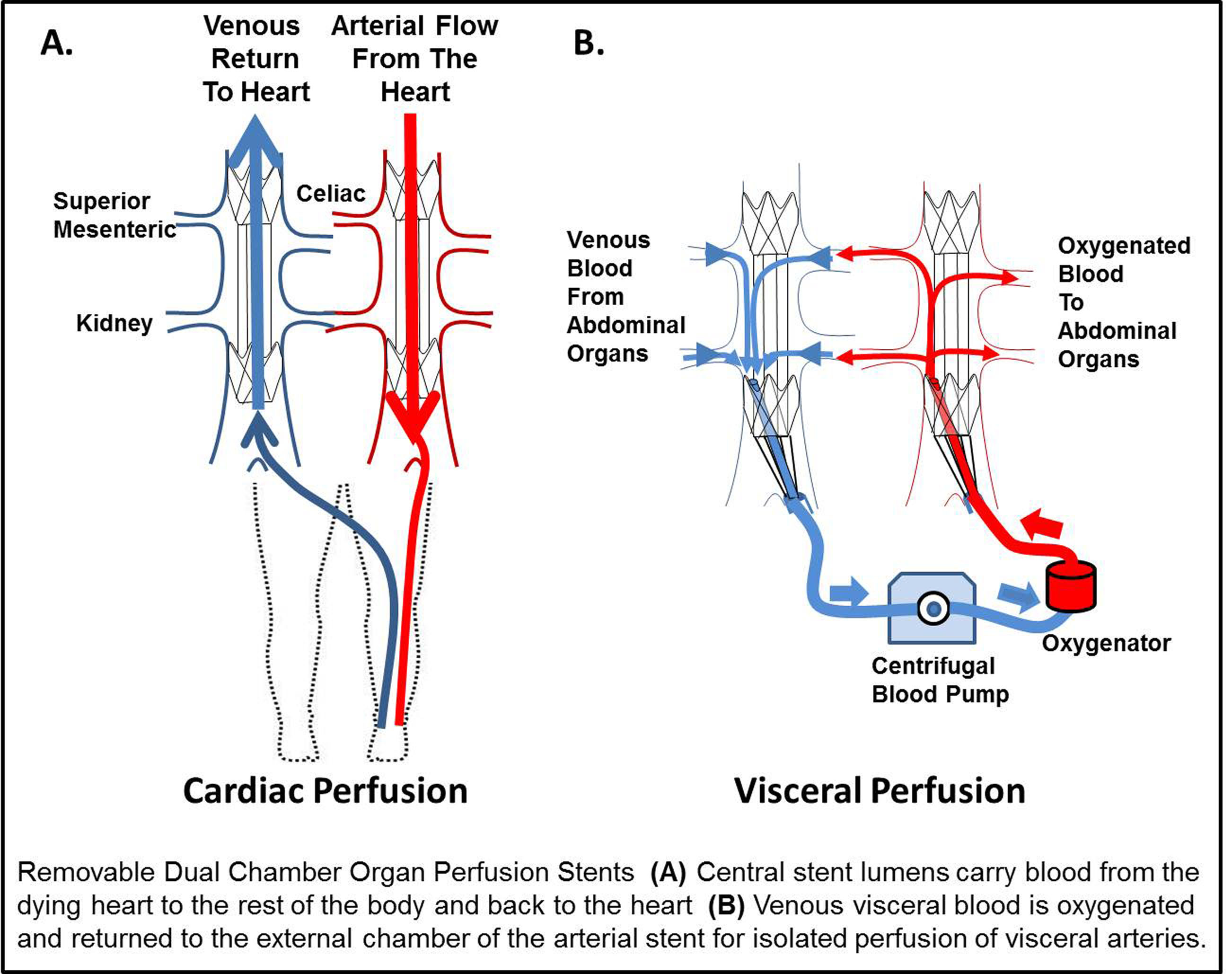
Introduction: The single most significant obstacle facing organ transplantation remains the critical shortage of donor organs. While donation after cardiac death (DCD) has demonstrated significant growth, these organs have inferior outcomes compared to other types of donation. During the ‘agonal period’, after life support is discontinued, malperfusion of organs leads to ischemic organ damage. As a result of the tenet that cardiac death should not be expedited, previous approaches could not be used until after cardiac death and after organ injury. In contrast to the global withdrawal of life support in DCD, among non-donors end of life care is determined by the family for individual organ systems. We have designed a dual chamber organ perfusion stent (OPS) to reduce ischemic injury by isolation of the visceral vessels and to perfuse only those organs with oxygenated blood while the heart fails on its own terms. This differs from all prior strategies because this removable stent would be placed by a needlestick femoral access and would allow the failing heart to continue to perfuse the rest of the body while protecting against ischemic injury of the visceral organs.
Methods: Prototype OPS were constructed from commercial stent components and final versions were custom made from nitinol and electrospun polyurethane. An OPS (n=5) was evaluated in a porcine model compared to non-stented controls. Paired venous and arterial OPS were deployed. Next, venous blood from the viscera in the external chamber was oxygenated and cycled to the external chamber of the arterial stent. Blood from the heart perfused the body through the large central lumen of the arterial OPS. DCD conditions were simulated for one hour with profound hypotension (SBP < 50 mm Hg).
Results: Invasive cardiac monitoring revealed no significant change in heart rate, mean arterial pressure, mixed venous oxygen, or venous pressure during stent deployment (P< 0.05). A small decrease was observed in the cardiac output consistent with exclusion of the visceral blood flow. Visceral blood flow of over 650 mL/min was achieved with the OPS and oxygen pressures were increased nearly 5 fold compared to agonal control animals. Control organs demonstrated severe malperfusion, contrasting to the well perfused OPS organs.
Conclusion: This pilot study suggests that an OPS offers: 1) rapid endovascular delivery 2) minimal impact on cardiac physiology 3) improved oxygen delivery to organs. Our early results suggest this novel stent design may increase availability of donor organs without impacting the natural cardiac death of the consented donor. Further development of this approach may ensure that nearly every consented donor successfully donates lifesaving organs.