C. Capriccioso1, J. Brennan1, B. Heiden1, A. Hage1, A. Zheutlin1, M. S. Sabel1 1University Of Michigan,Surgery,Ann Arbor, MI, USA
Introduction: The metric of success for neoadjuvant chemotherapy (NACT) in breast cancer has traditionally been a complete pathologic response (pCR). Consequently, NACT is considered less often in luminal A patients, where a pCR is unlikely. However, a pCR is not always necessary to allow avoidance of mastectomy. We sought to examine the impact of NACT on BCT eligibility based on histologic subtype, particularly among patients with the luminal A subtype.
Methods: Our IRB-approved prospective breast cancer database was queried for patients who underwent NACT prior to curative surgery. Charts were reviewed for clinicopathologic features, pathologic response, surgical therapy and BCT eligibility before and after NACT as determined by the surgeon. Patients with inflammatory breast cancer or eligible for BCT prior to NACT were excluded when calculating the conversion rate. Patients were categorized by molecular subtype based on estrogen receptor, progesterone receptor and Her-2/neu overexpression: luminal A (ER or PR positive, Her-2/neu negative), luminal B (ER or PR positive, Her-2/neu positive), HER2- enriched (ER/PR negative, Her-2/neu positive), or basal –like (triple negative).
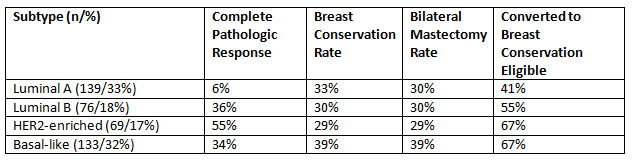
Results: Of the 417 patients identified, 139 (33%) were luminal A, 76 (18%) were luminal B, 69 (17%) were HER2-enriched and 133 (%) were triple negative. Rates of pCR were highest (55%) among HER2-enriched patient and were extremely low (6%) among luminal-A patients. The breast conservation rate was relatively similar across subtypes, ranging from 29% to 39%. However, the BCT rate was influenced by NACT use among initially BCT-eligible patients, and by BCT-eligible patients after NACT opting for bilateral mastectomy. Among non-inflammatory patients determined to be ineligible for BCT prior to NACT, conversion to BCT-eligibility (regardless of subsequent surgery) was highest (nearly 2/3rd) for HER2-enriched and triple-negative patients. While the BCT conversion rate was lower for hormone receptor positive, HER2-negative patients (41%), there was still a substantial impact on surgical outcomes.
Conclusion: For NACT, pCR rates or BCT rates present an incomplete assessment of this therapy by overlooking the impact on surgical decision making. A substantial percentage of luminal A patients can avoid mastectomy with NACT, despite a significantly lower pCR rate. Measuring BCT rates after NACT does not adequately measure the impact of NACT on BCT eligibility, as NACT may be used in BCT-eligible patients, and after NACT, BCT-eligible patients may opt for unilateral or bilateral mastectomies. Surgical response rate, or conversion to BCT eligibility, as assessed by the surgeon, should be measured prospectively in future trials of neoadjuvant chemotherapy.