A. M. Steely1,2, T. Mann-Gow2, B. S. Childs2, M. Kida2,3, P. Zvara1,2 1The University Of Vermont Medical Center,Department Of Surgery,Burlington, VT, USA 2The University Of Vermont,College Of Medicine,Burlington, VT, USA 3The University Of Vermont Medical Center,Department Of Pathology And Laboratory Medicine,Burlington, VT, USA
Introduction: Neointimal hyperplasia remains a common problem following vascular surgery. Current murine mechanical injury models, which denude the endothelium in a retrograde fashion and require suture ligation of some portion of the arterial tree, have been criticized for the small proliferative response and high inter-animal variability. Our aim was to develop a simple, reproducible, mechanical, antegrade murine common carotid artery (CCA) neointimal hyperplasia model that does not require suture ligation of any portion of the cervical arterial tree.
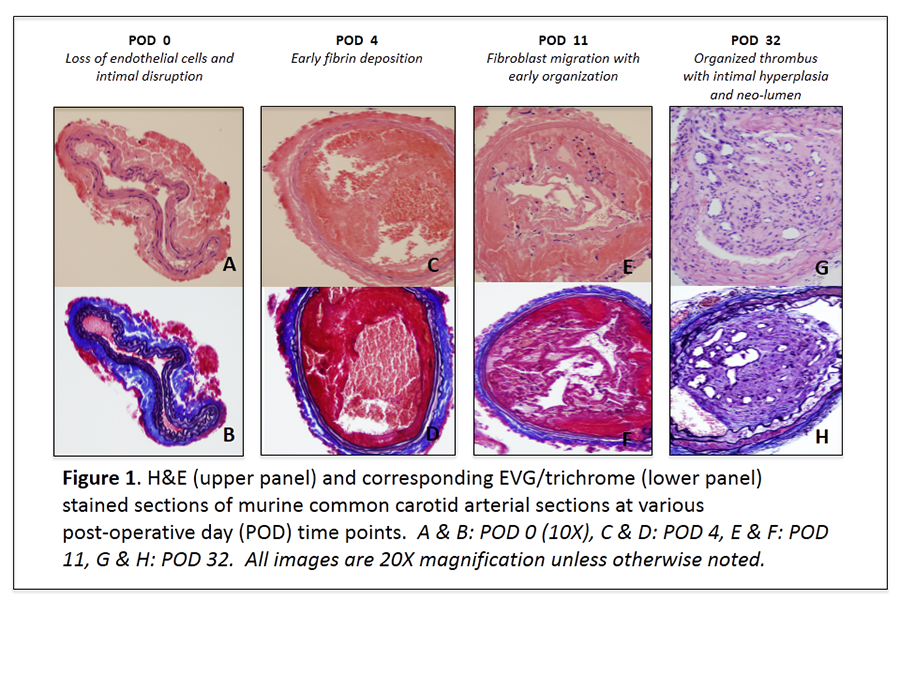
Methods: After creating an arteriotomy in the CCA with a 30G needle, a 0.010-inch hydrophilic wire was used to cannulate the CCA via the arteriotomy and denude the endothelium in an antegrade fashion. Hemostasis at the arteriotomy site was achieved by placing a topical cellulose matrix (Surgicel, Ethicon, Inc. Somerville, New Jersey) over the arteriotomy site. Mice were euthanized 0, 4, 11, and 32 days following CCA injury after systemic formaldehyde perfusion fixation. CCA sections were stained with hematoxylin and eosin (H&E), trichrome, and elastin van Gieson (EVG).
Results: Loss of endothelial cells and intimal disruption was observed at POD 0. Early fibrin deposition was seen in the injured arterial segment at POD 4, however, fibroblast migration with early organization was not observed until POD 11. Organized thrombus with intimal hyperplasia and neo-lumen formation was evident on POD 32.
Conclusion: Antegrade mechanical injury with a hydrophilic wire effectively and reproducibly denudes the endothelium of the murine CCA with subsequent neointima formation throughout the injured arterial segment. This novel, physiologic, and reproducible CCA injury model may aid in the development of innovative pharmaceuticals to prevent and treat neointimal hyperplasia.