M. Schmidt1, G. Loganathan1, S. Narayanan1, A. Jawahar1, J. Pradeep1, M. G. Hughes1, S. K. Williams1, B. N. Appakalai1 1University Of Louisville,Department Of Surgery,Louisville, KY, USA
Introduction: Islet transplantation is one of the best approaches to restore insulin independence in Type-1 diabetic patients. Generally, the transplant recipients achieve insulin independence, however, the transplanted islets undergo cell death due to poor blood supply immediately after transplantation. Native islets in the pancreas have a rich vascular supply however, the current islet isolation technique completely severs the vasculature to islets. As such, these islets become avascular and many of them die immediately after transplantation. The creation of blood vessels around islets (Bioengineered islets –BEI) will enable islet cell survival and reverse diabetes efficiently. Our specific aim was to stimulate intra-islet endothelial cells (IIEC) to form peri-islet capillaries (PIC) in culture prior to transplantation. We tested our hypothesis and accelerated the formation of PIC to human islets by stimulating the IIEC with the addition of ECGS (a mixture of endothelial growth factors) in a 3D-culture system.
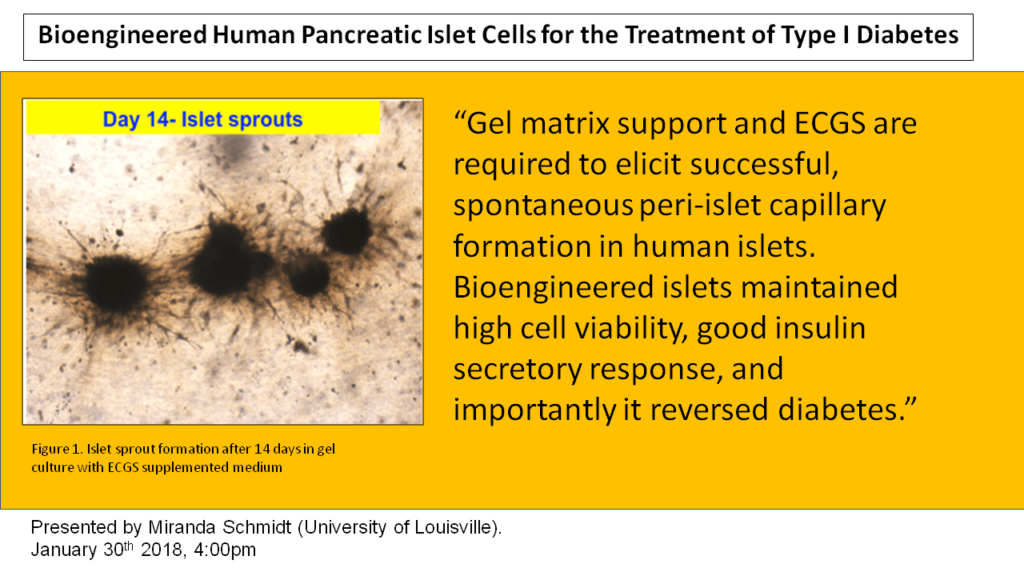
Methods: Human islets cells were isolated from brain-dead donor pancreases (n=15) and the islets (200 per well) were cultured in a 3D system using rat-tail collagen-I gel with ECGS containing medium for 14 days. 2D cultured islets and 3D cultured islets without ECGS were used us control groups. Time-lapse microscopy (Cytation 5) was used to monitor islet-derived PIC growth every day for 14 days. After the sprout formation, human endothelium was identified via labeling with UEA-1 staining. To study diabetes reversal, the BEI were implanted in to diabetic nude mice at the subcutaneous site.
Results: These results indicate this approach induced cellular sprouting from human islets and were positive for endothelial cells. The bioengineered islets maintained >90% cell viability, high insulin secretory capacity when compared to control islets and importantly, BEI reversed diabetes in mice. Naked islets cultured in a 2D environment did not form any sprout. However, the presence of ECGS and 3D-culture system induced cellular sprouting from human islets within 7 days. UEA-1 FITC Green and RedDot 2 labeling indicated that several of these sprouts were endothelial. We speculate that our isolated islets gave rise to peri-islet sprouts. 3D-cultured islets maintained >90% cellular viability after 14 days, whereas 2D-cultured islets displayed disintegration of their borders as well as reduced viability. Statistically significant difference (P<0.05) was observed in number of sprouts when islets were cultured in 3D environment without ECGS.
Conclusion: We demonstrated that 3D matrix support and ECGS are required to elicit successful, spontaneous PIC formation in human islets. EGCS solution is essential for the stimulation of PIC. ECGS with 3D culture may provide unique avenues to accelerate islet neovascularization following transplantation. We conclude that creating BEI is a novel approach for the treatment of type-I diabetes.