A. Yeh1, E. Conners2, B. Firek2, M. B. Rogers2, R. Cheek2, M. J. Morowitz2 1University Of Pittsburgh,Department Of Surgery,Pittsburgh, PA, USA 2Children’s Hospital Of Pittsburgh Of UPMC,Division Of Pediatric General And Thoracic Surgery,Pittsburgh, PA, USA
Introduction:
Critically ill patients have significantly deranged gut microbiota that may promote gastrointestinal and systemic inflammation. One major contributor to dysbiosis is the use of chemically-defined enteral formulas for nutrition that are composed of high sugar, low fiber, and artificial ingredients. Whole-food enteral formulas represent a healthy alternative that lacks these shortcomings. We hypothesize that whole-food enteral formulas will restore a healthy gut microbiota and reduce dysregulated inflammation in critically ill patients. To test this hypothesis, we used the DSS-induced colitis model in mice as a surrogate for the gut inflammation seen commonly during critical illness.
Methods:
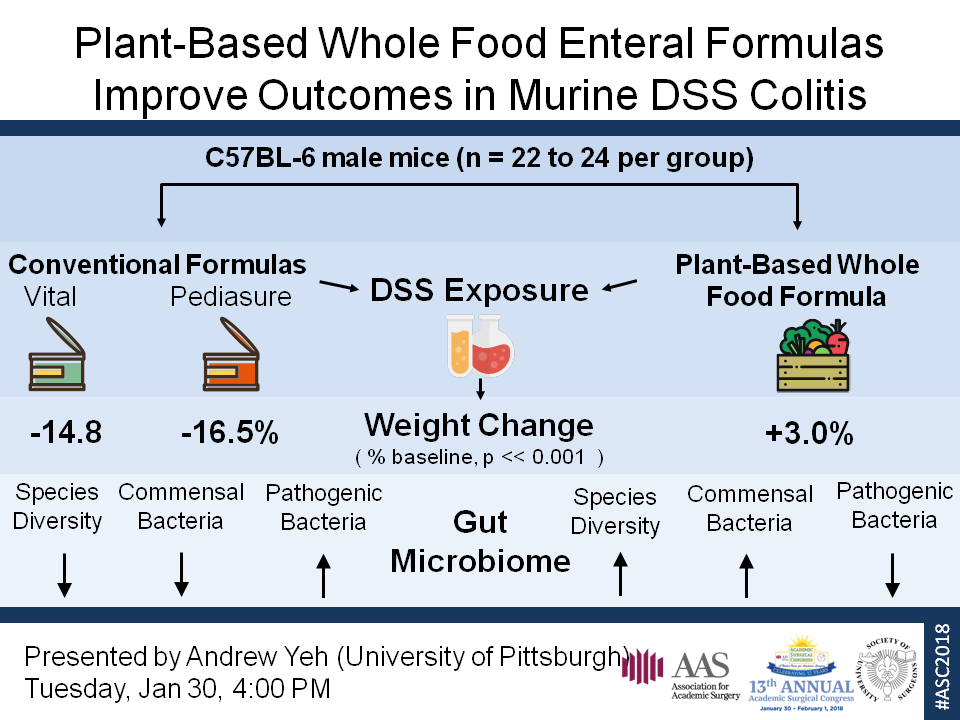
C57BL/6 mice were fed standard chow, chemically-defined formulas Vital (V) or Pediasure (P; Abbott Nutrition), or plant-based whole-food formula Liquid Hope (LH; Functional Formularies) for 7 days. Mice were then given 4% DSS water or control water for 4 days. Weight and disease activity indices (DAI) were measured daily. Upon sacrifice, plasma was obtained for IL-6 analysis and colon length was measured as markers of inflammation. Stool samples were collected before starting the diet, after 7 days on the diet/before starting DSS water, and after DSS exposure. Bacterial 16S rRNA gene sequences in each sample were amplified, sequenced on the Illumina MiSeq, and analyzed with QIIME.
Results:
Weight loss was more severe (p<0.01) and DAI’s were higher (p<0.01) after DSS exposure in mice fed P or V (n=16 per group) compared to LH. IL-6 plasma levels and colon length were also significantly increased (p<0.01) and decreased (p<0.01), respectively, in mice fed V and P compared to LH indicating worse inflammation. Gut microbiome analysis demonstrated reduced species diversity and altered species composition in mice fed V and P compared to LH (p<0.05). Prior to DSS exposure, mice fed V and P compared to LH contained greater abundance of Enterobacteriaceae (p<0.05), a family containing Gram-negative pathogens associated with gut inflammation, and lesser abundance of Clostridiales (p<0.05), an order containing many commensal bacteria associated with immune homeostasis.
Conclusion:
LH significantly improved outcomes in mice exposed to DSS compared to standard formulas, V and P. LH also prevented gut dysbiosis, maintaining healthy commensal organisms, and preventing invasion of pathogenic organisms. Future work will need to determine if direct effects of the diet composition and/or indirect effects on the microbiome are responsible for the protective effect of whole-food nutrition, and whether its use in the ICU can improve outcomes for critically ill patients receiving enteral nutritional support.