N. Cupitra1, A. Múnera Duque2, JP León2, R. Narvaez-Sanchez1 1PHYSIS Group, Faculty of Medicine, University of Antioquia, Medellín, Antioquia, Colombia 2Department of Surgery, Faculty of Medicine, University of Antioquia, Medellín, Antioquia, Colombia
Cancer is one of the leading causes of death on the planet: in 2012, 8.2 million deaths were attributed to it, with the highest number of victims caused by lung cancer (19, 4%), hepatic (9.1%), gastric (8.8%) and colorectal (8.4%); (1). As an abnormal growth of cells, cancer needs vascular changes for growing and metastases. In the last two decades the tumor vascular system and its possible role in the development of cancer has been investigated, proposing it as a target for the control of nutrient supply, by modification of vascular tone, angiogenesis and neovascularization (2). Vascular reactivity (VR) could be a limiting factor for growth in cancer, since growing tissues require vasodilation and new vascularization. Promoting vasoconstriction in the tumor could reduce the supply of nutrients, while promoting vasodilation could increase therapies to reach more cancer cells. The present work aims to quantify differences in human VR of tumoral (TU, arteries which directly irrigate the tumor), extratumoral (ET, arteries of a tumoral patient, which do not irrigate tumor but the distal and proximal colon near the tumor) and non-tumoral arteries (NT, branches of mesenteric circulation of colectomized patients due to a non tumoral pathology), to the stimulus of potassium chloride (KCl), phenylephrine (FE), ET-1, U-46619 (analog of thromboxane A2), and bradykinin (BK).
Six patients with colon cancer and two with non tumoral pathology, from 30 to 80 years old, underwent hemicolectomy at “IPS Universitaria Leon XIII” Hospital, in Medellin, Colombia. All patients signed the respective informed consent. Mesenteric branches were collected and preserved in Krebs-Henseleit solution (SK, composition (mEq/liter): Na+, 143.5, K +, 5.4, Ca2 +, 5.1, Mg2+, 2.4, Cl-, 128, H2PO4-, 1.2, HCO3, 24.9, SO42-, 2.4, and glucose, 10) at 4° C where excess of peripheral tissue was removed. The arterial segment was cut into rings 3-5 mm long, which were mounted on stainless steel hooks in parallel through the lumen. One of the hooks was attached to a force transducer (ADInstruments Ltd, Model MLT0201, UK), and the other was held as a fixed support. The preparation was mounted in a 10 ml organ bath (Panlab/ Harvard, Model LE01046, Spain) containing SK. The tissue was maintained at 37° C and bubbled with carbogen (95% O2- 5% CO2). Each preparation was stabilized at 2 grams of basal tension along 90 minutes, before initiating stimulations with agonists, changing SK every 20 minutes. After stabilization the arterial ring was stimulated twice with KCl 4×10-2 M recording the response for 10 minutes, and washing for 20 minutes between stimuli. Then U-46619 1×10-7 M and BK 1×10-5 M were applied to confirm the presence of endothelium. This verification was performed on all arterial rings. Then the evaluation was done applying in some rings KCl at a single dose KCl 4×10-2 M, or FE at cumulative dose of 5×10-8 M to 1×10-5 M, or U-46619 cumulative dose of 1×10-13 M to 1×10-7 M, or, precontracting with U-46619, BK at cumulative dose of 1×10-9 M to 1×10-5 M. The maximal relative response (“Emax%KCl”, maximal contraction normalized in respect to maximal contraction to KCl) and sensitivity (EC50, concentration which generates 50% of response) were recorded by the Powerlab PL-3504 polygraph (ADInstruments, Australia). Analyzes were performed with t-Student and ANOVA with significance level p <0.05. The EC50 was calculated by non-linear regression of the concentration-response curves. The statistical package GraphPad prism 6 Statistics was used.
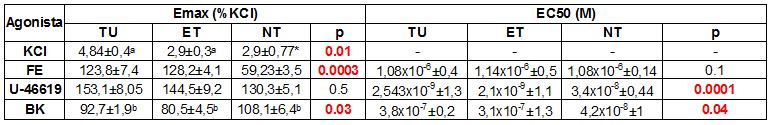
Experimental results are presented as means ± SEM of the Emax and EC50:
The greater response of TU but not of NT to KCl suggests histological differences that we have not specified yet. The increase in Emax to FE and EC50 U-46619 of TU and ET with respect to NT, suggests that the tumor microenvironment favors the contractile response in the arteries that irrigate it: it has been reported that oxidative stress activates kinases that promote proliferation and resistance to anticancer agents, and induces endothelial dysfunction that worsens vasodilation. The beta-adrenergic receptors participate in the initiation and progression of the tumor; but more evidence is lacking about the role of alpha-adrenergic signaling and thromboxane A2 in vascular control in cancer. The higher sensitivity to U-46619 in TU and ET suggests an increase in the activity of the thromboxane-A2 dependent mechanisms, coinciding with what is reported in greater activation of thromboxane-prostanoid receptors. Our relaxation analysis did not show differences of VR to BK in Emax nor in EC50 between TU and ET, contradicting a possible endothelial damage, at least in TU and ET. These preliminary results require contrasting with non-tumoral arteries, measuring differential expression of receptors, doing histology evaluating integrity of the vascular wall, and quantifying probable endothelial dysfunction by specifying affected mechanisms, using specific blockers.