C. H. Quinn1, R. Uhlich1, A. M. Beierle1, R. Marayati1, J. E. Stewart1, J. M. Coleman2, V. R. Atigadda3, G. K. Friedman4, E. A. Beierle1 1University of Alabama Birmingham,Division Of Pediatric Surgery, Department Of Surgery,Birmingham, ALABAMA, USA 2University Of Alabama at Birmingham,Department Of Neurosurgery,Birmingham, Alabama, USA 3University Of Alabama at Birmingham,Department Of Dermatology,Birmingham, Alabama, USA 4University Of Alabama at Birmingham,Division Of Pediatric Hematology Oncology, Department Of Pediatrics,Birmingham, Alabama, USA
Introduction: Oncolytic virotherapy is a promising emerging therapeutic option for difficult to treat cancers. Combination approaches to maximize the oncolytic effect are being explored. Neuroblastoma, a solid tumor of the nervous system, is responsible for over 15% of pediatric cancer deaths. We have demonstrated significant neuroblastoma oncolysis with genetically engineered oncolytic Herpes Simplex Virus (oHSV) M002. Current therapy for neuroblastoma includes treatment with retinoic acid (RA) to stimulate tumor cell differentiation. We have found that UAB30, a novel RXR agonist, has similar cell differentiating properties as RA and is comparably efficacious in preclinical studies with neuroblastoma. We hypothesized that UAB30 would alter the tumor cell profile through alternate gene expression and increase tumor susceptibility to M002, thereby enhancing the efficacy of these potential neuroblastoma treatments.
Methods: M002 is a genetically engineered oHSV with deleted copies of both ?γ134.5 loci and UAB30 is a 9-cis-RA analogue. Neuroblastoma cell lines SK-N-AS (As) and SK-N-BE(2) (Be) were used. Single step and multistep viral recovery assays were performed following 72 hour pre-treatment with increasing doses of UAB30 to investigate M002 total viral production and replication rates, respectively. Flow cytometry was utilized to analyze expression of cell surface viral receptors following UAB30 pre-treatment. Cell viability studies were measured with alamarBlue® and synergy analyzed using the method of Chou and Talalay.
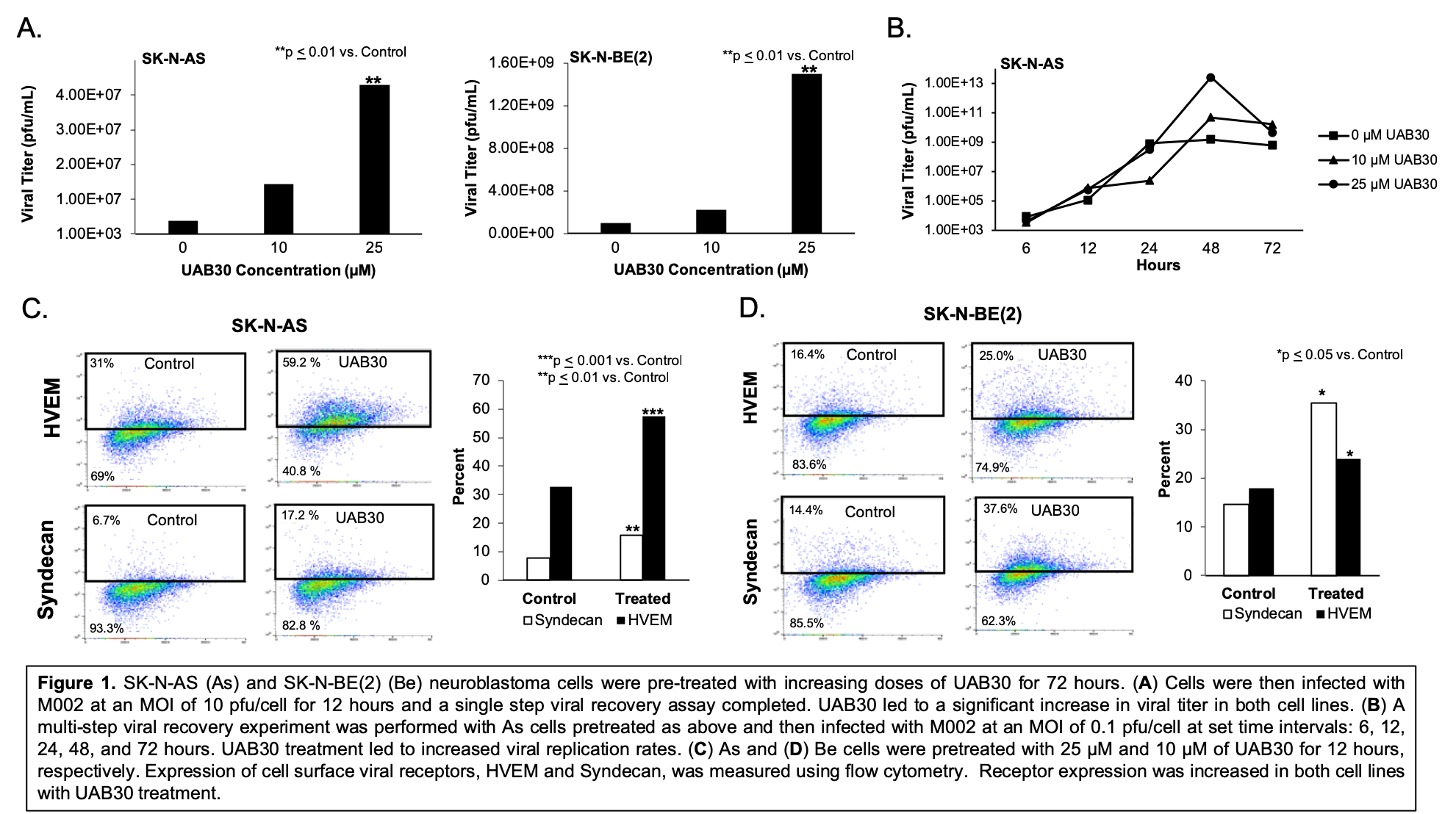
Results: Increasing doses of UAB30 significantly increased M002 viral titer (Figure 1A). 25 µM of UAB30 resulted in significantly increased M002 replication over time (Figure 1B). Cell surface HSV entry receptors, Syndecan and HVEM, were upregulated following UAB30 treatment (Figure 1C, D). In vitro studies with As cells treated with M002 and 25 µM of UAB30 resulted in a 7.4-fold decrease in the lethal dose required to kill 50% of cells compared to M002 alone (0.12 plaque forming units (PFU)/cell and 0.89 PFU/cell, respectively), indicating synergy.
Conclusions: UAB30 effectively increased M002 viral titer and replication. UAB30 combined with M002 resulted in significantly more neuroblastoma cell death compared to M002 alone. UAB30 increased the expression of several HSV cell entry receptors, providing a potential mechanism for the synergy seen between the agents. These studies suggest that UAB30 and M002 together may provide improved efficacy in neuroblastoma and validate the need for continued investigation.