K. Bellam1, D. D. Harris1, M. Broadwin1, S. A. Sabe1, C. Stone1, M. Kanuparthy1, R. Abid1, F. Sellke1 1Brown University School Of Medicine, Cardiothoracic Surgery, Providence, RI, USA
Introduction: Our recent studies using porcine models have demonstrated that serum-starved extracellular vesicles (EVs) mitigate inflammation in animal models of acute myocardial ischemia/reperfusion and improve blood flow via inducing collateral vessel growth in ischemic myocardium. Previously, our lab demonstrated that EVs have increased abundance of antioxidant, pro-angiogenic, anti-inflammatory, and pro-survival proteins in ischemic tissues, but have yet to be investigated in nonischemic tissues. This study investigates the effect of hypoxia-modified EV therapy on oxidative stress and inflammatory signaling in nonischemic territories in a porcine model of chronic myocardial ischemia.
Methods: Human bone marrow-derived stem-cells (HBMSCs) were cultured to 80% confluence before being placed in a humidified hypoxia chamber. The media was collected and the EVs were isolated and characterized with electron microscopy, nanoparticle tracking analysis, and immunoblotting as previously described and published. At 11 weeks of age, Yorkshire swine underwent placement of an ameroid constrictor to the left circumflex artery to induce chronic myocardial ischemia. Two weeks later, the pigs underwent redo-left thoracotomy for an intramyocardial injection of either saline (Control, n = 7) or hypoxia-modified EVs (HEV, n = 7) into the ischemic ventricular territory. Five weeks later, pigs were euthanized, and left ventricular myocardial tissue was harvested. Areas of ischemic (left circumflex territory) and nonischemic (left anterior descending territory) left ventricular myocardial tissue were determined by microparticle perfusion analysis. Protein expression was measured with immunoblotting.
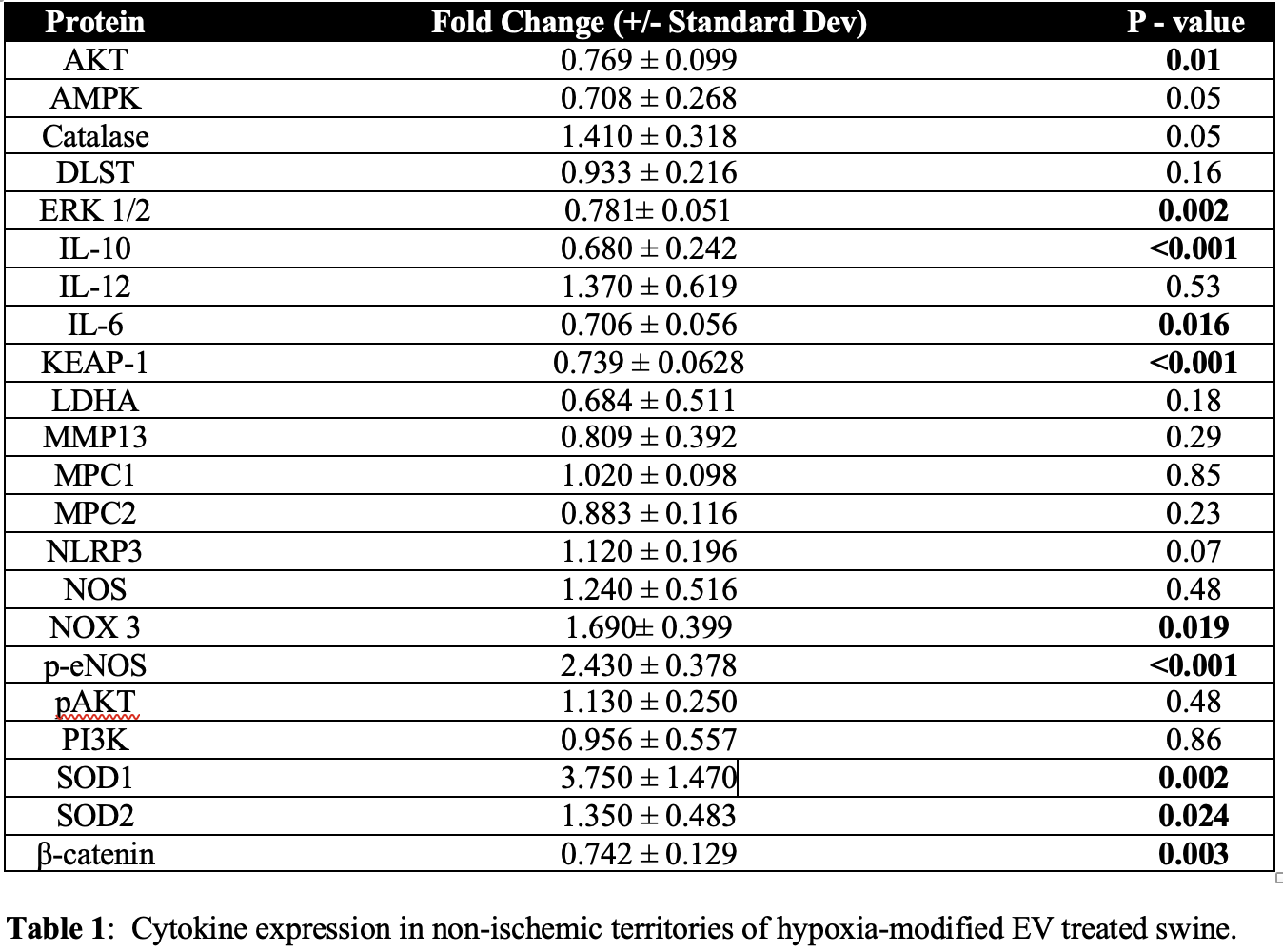
Results: Hypoxia-modified EV treatment was associated with increased expression of the anti-inflammatory protein IL-10 and antioxidant proteins p-eNOS, SOD1, and SOD2 in nonischemic myocardial territories compared with the control (all p<0.05). Treatment was also associated with decreased expression of inflammatory protein β-catenin (p=0.003), pro-oxidant protein KEAP-1 (p<0.0001), ERK 1/2 (p=0.002), AKT (p=0.008, and IL-6 (p=0.016). No change was seen in proteins p-AKT, PI3K, total NOS total (all p>0.05).
Conclusion: Nonischemic territories in the hypoxia-modified EV group had increased expression of anti-inflammatory and anti-oxidative stress markers compared to the control saline group in the setting of induced chronic myocardial ischemia. This study provides insights into the local regional effects of EVs in areas adjacent to the injection site, furthering our knowledge of its role as a potential therapeutic in human disease.