M. E. Jones-Carr1, J. Perry1, R. D. Reed1, C. Killian1, G. Baker1, P. Porrett1, D. J. Anderson1, V. Kumar1, A. Shunk2, J. E. Locke1 1University Of Alabama at Birmingham, Heersink School Of Medicine, Department Of Surgery, Division Of Transplantation, Birmingham, Alabama, USA 2Legacy of Hope, Birmingham, ALABAMA, USA
Introduction:
Medical treatments which lack regulatory approval often rely on cell lines or animal models, which can differ substantially from in vivo conditions, and as such a pre-clinical human model is needed. Brain death, defined as irreversible cessation of all functions of the entire brain, including the brain stem, is traditionally a means for organ donation. However, brain-dead decedents are often precluded from organ donation due to various health factors, affording the opportunity to study brain death as a novel preclinical human model for the study of disease treatments particularly non-procurement surgical interventions. Herein, we describe a new pre-clinical human model, the brain-dead decedent: The Parsons Model.
Methods:
Brain-dead decedents that were unable to donate their organs for the purposes of transplantation were identified by our local organ procurement organization and subsequently enrolled after family consent. A total of four adult decedents were enrolled; the second decedent was excluded from surgical intervention due to a leukemic blast crisis. Decedents were maintained on the ventilator in a critical care setting. Hemodynamic and physiologic data were recorded. They underwent bilateral native nephrectomies and bilateral porcine kidney xenotransplants.
Results:
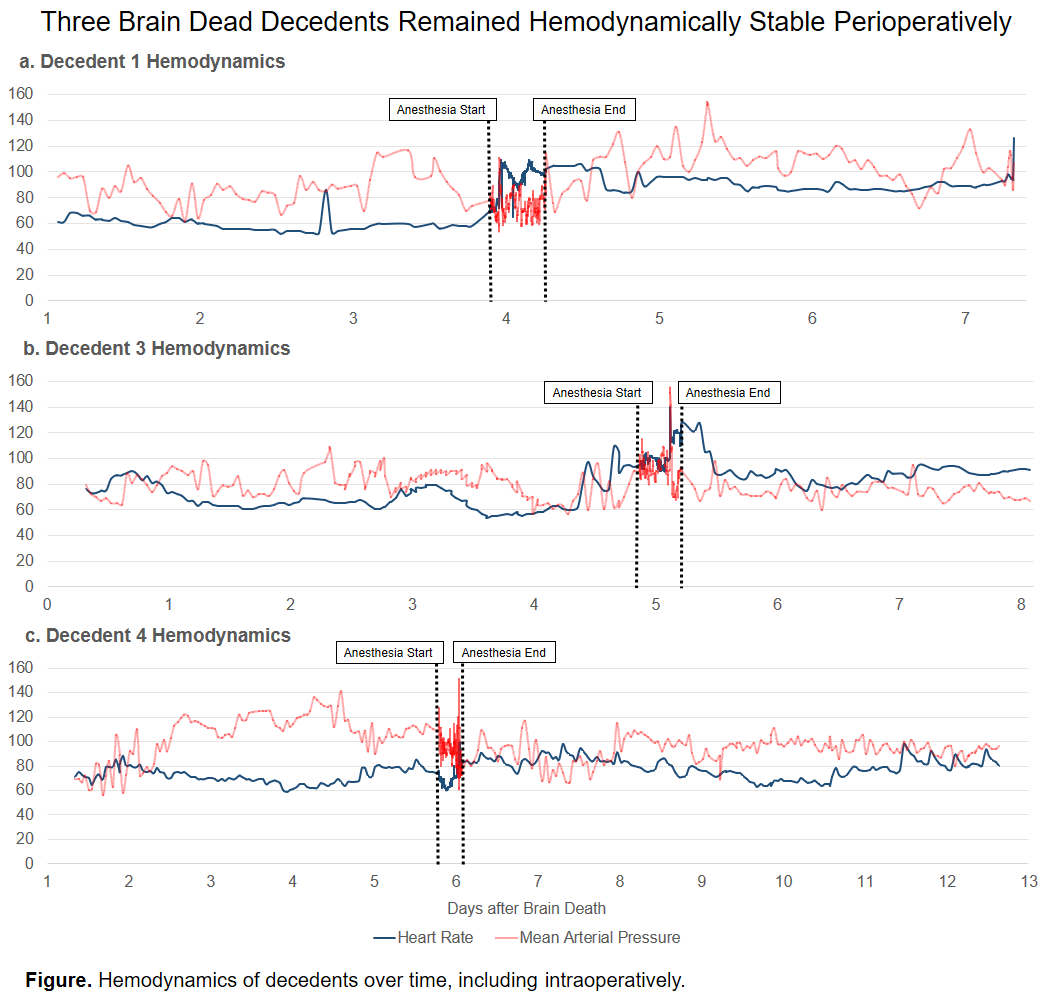
All decedents were male with ages ranging from 53-66 years. Decedents underwent xenotransplantation 3.9, 4.9, and 5.8 days after their first exam documenting brain death, and were supported for a total of 7.3, 8.1, and 12.8 days after brain death. The first and third decedents were mildly bradycardic pre-operatively. All decedents were on vasopressor infusions pre- and intraoperatively. Postoperatively the first required ongoing vasopressor infusions, as did the third and fourth intermittently. The third and fourth decedents required antihypertensives sporadically throughout their course. All decedents had transient hypertension with xenograft reperfusion. The second decedent was also tachycardic with reperfusion; this quickly subsided with beta blockade.
Conclusion:
We report the first series with a novel preclinical human model for the study of surgical interventions, henceforth known as the Parsons Model. Our decedents’ hemodynamics supported major non-procurement surgical interventions in the setting of brain death. The brain-dead decedent model could facilitate innovation of new medical devices, surgical interventions, or even surgical education.