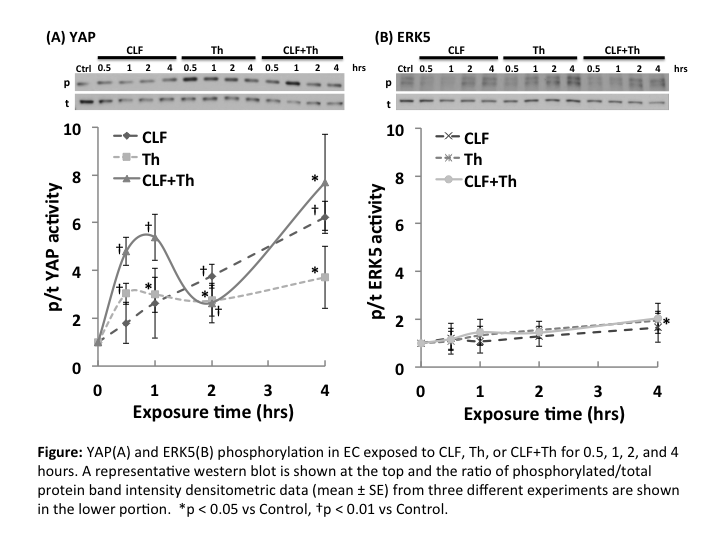
J. Kurita1,2, G. Chitragari1,2, B. E. Sumpio1,2 1Yale University School Of Medicine,Vascular Surgery,New Haven, CT, USA 2VA Connecticut Healthcare System,Vascular Surgery,West Haven, CT, USA
Introduction: The endothelium can be activated not only by mechanical forces of the circulation but also by circulating chemicals such as thrombin (Th). However, the effect of combined mechanical and chemical stimulation of endothelial cells (EC) is poorly understood. The purpose of this study was to investigate the effect of steady laminar flow (CLF), as a mechanical stimuli, and Th, as a chemical stimuli, on yes-associated protein (YAP) and extracellular signal-regulated kinase 5 (ERK5) activities in EC. YAP is a mechano-signaling protein that controls growth and proliferation depending on cell shape. ERK5 is an intracellular protein that maintains EC integrity.
Methods: Bovine aortic ECs seeded on fibronectin coated glass slides were grown to confluence in culture medium containing 10% fetal bovine serum. Thereafter, they were serum starved for 16 hours and were subjected to 0.5, 1, 2, or 4 hours of CLF utilizing a parallel plate flow chamber system controlled by a computerized pump for 4 hours at 37? in the presence or absence of Th (4U/ml). Western blot analysis of total and phospho-YAP and ERK5 was performed. The activities of YAP and ERK5 were determined based on the ratio of immunoblot intensity of phosphorylated to total protein. Fold change compared to static control (Ctrl) at 0 hour was calculated and compared for significant difference using t-test. P value of <0.05 was considered statistically significant.
Results: ERK5 activity rose slightly through 4 h for all experimental groups but was only significantly increased in the Th group at 4 hours (1.95±0.39; p<0.03 vs Ctrl) (Figure B). YAP was phosphorylated by Th and the combination of CLF+Th (2.96±0.73; p<0.01 and 2.44±1.16; p<0.05 vs Ctrl, respectively). YAP phosph/total ratio was significantly increased with time through 4 h in the CLF group (p<0.05 vs Ctrl), while the ratio in the CLF+Th group showed the similar time course pattern as in the Th group within 2 hours. However, at 4 h it rose to the level of the CLF groups (Figure A).
Conclusion: Thrombin and flow did not activate ERK5. YAP was phosphorylated in EC exposed to both thrombin and flow although flow stimulation was stronger. The combination of flow and thrombin had an additive effect on YAP phosphorylation. These results indicate selective activation of intracellular proteins by mechano-chemical signals. Further studies have to be done to elucidate the interactions of these signaling pathways and to understand their roles in endothelial response to stimuli.