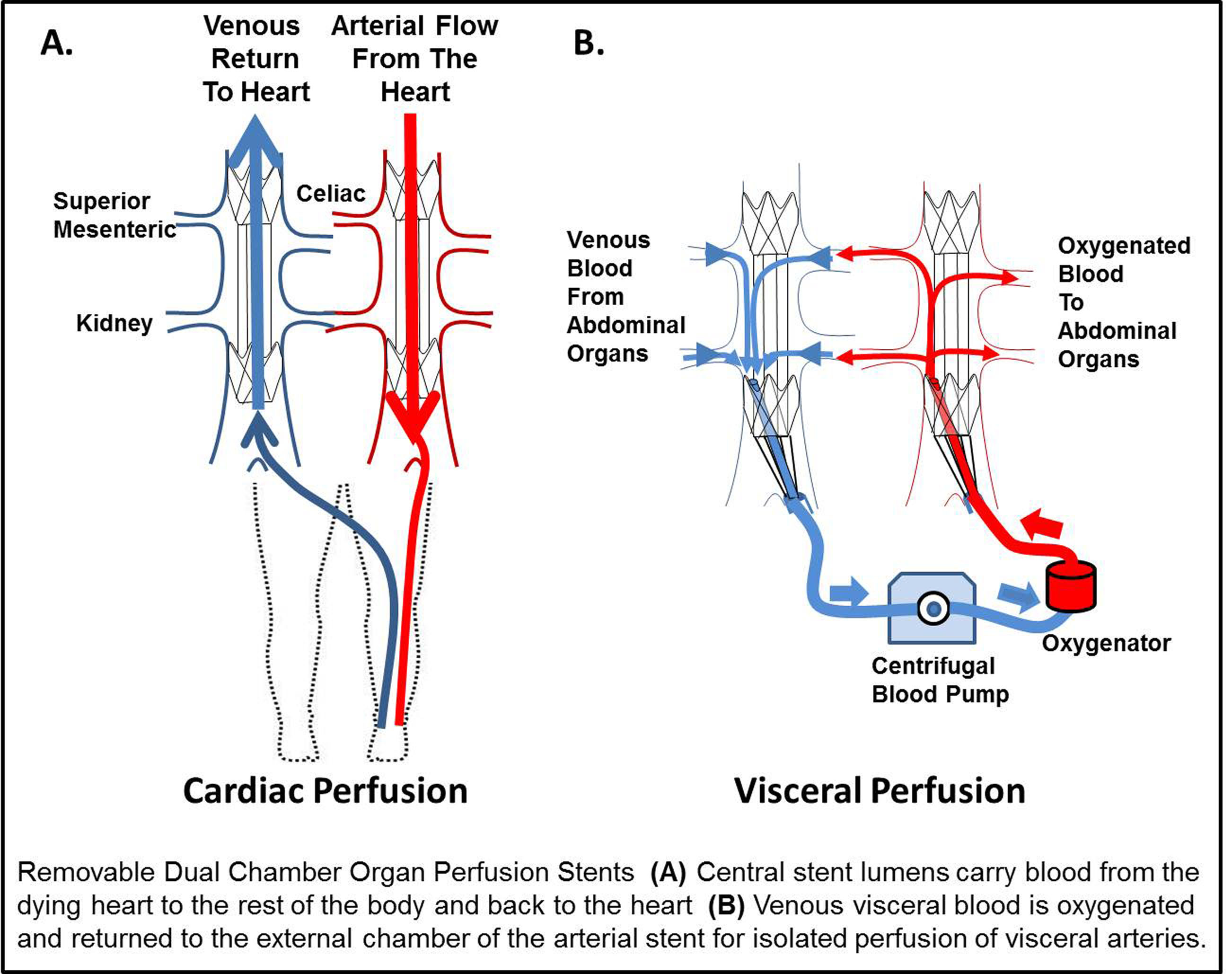
T. Horiuchi1,2, H. Shiba1, N. Saito1,2, Y. Shirai1,2, R. Iwase1, K. Haruki1, Y. Fujiwara1, K. Furukawa1, T. Uwagawa1, T. Ohashi2, K. Yanaga1 1The Jikei University School Of Medicine,Department Of Surgery,,Tokyo, , Japan 2The Jikei University School Of Medicine,Department Of Gene Therapy, Research Center For Medical Science,Tokyo, , Japan
Introduction: Pancreatic cancer has a poor prognosis among visceral cancers, with an overall 5-year survival rate of around 5%. Recently, gemcitabine plus nab-paclitaxel therapy was reported to be superior to gemcitabine alone in patients with advanced pancreatic cancer. However, the tumor resistance to chemotherapy is common and activation of nuclear factor-kappa B (NF-κB) plays a key role in attenuation of anti-tumor effect of chemotherapy in pancreatic cancer. We previously reported that nafamostat mesilate, a synthetic serine protease inhibitor, inhibited NF-κB activation and induced antitumor effects for pancreatic cancer. We hypothesized that nafamostat mesilate downregulates the activity of NF-κB and improves therapeutic outcome of pancreatic cancer.
Methods: In vitro, we assessed NF-κB activity, cell viability, induction of caspase cascade, and quantification of apoptosis of human pancreatic cancer cell lines (PANC-1, MIA PaCa-2, ASPC-1) in the following five groups: 1) gemcitabine and nab-paclitaxel, 2) nafamostat mesilate alone, 3) triple combination (gemcitabine, nab-paclitaxel and nafamostat mesilate), or 4) vehicle as control. In vivo, we established orthotopic pancreatic cancer model in mice by injection of PANC-1 cells to the pancreas. At six weeks after injection, the animals were treated with nafamostat mesilate 3 times a week (Nafamostat mesilate group), with gemcitabine plus nab-paclitaxel once a week (Chemotherapy group), or with combination of nafamostat mesilate 3 times a week and gemcitabine plus nab-paclitaxel once a week (Triple combination group). In Control group, only vehicle of gemcitabine and nafamostat mesilate were injected at the same time course.
Results:In vitro, NF-κB activity in Chemotherapy group was higher than that in Control group, and NF-kB activity was significantly surpressed in Nafamostat mesilate group and Triple combination group as compared with Control group and Chemotherapy group, respectively (PANC-1: p<0.001, MIAPaCa-2: p<0.001, ASPC-1: p<0.001). Cell viability in Triple combination group was lower than that in Chemotherapy group. Cleaved caspase-3 and 8 level in Triple combination group was the greatest in the 4 groups. Apoptosis in Triple combination group was significantly more frequent than that in Chemotherapy group. In vivo, tumor size in Tripe combination group was smaller than those in other groups. NF-κB activation of the excised tumor in Triple combination group was significantly inhibited in comparison with that in Chemotherapy group (p<0.01). Cleaved caspase-3 level and the number of apoptotic cells in the Triple combination group were the greatest in the 4 groups.
Conclusion:Combination chemotherapy of gemcitabine plus nab-paclitaxel with nafamostat mesilate exerts enhanced anti-tumor effect against orthotopic pancreatic cancer model in mice.