G. Brat3, B. Yorkgitis3, A. Seshadri3, J. Keegan3, J. Dolan3, C. Hauser2, A. Salim3, R. Askari3, J. Lederer3 2Beth Israel Deaconess Medical Center,Trauma Surgery,Boston, MA, USA 3Brigham And Women’s Hospital,Department Of Surgery,Boston, MA, USA
Introduction: Trauma induces a complex physiological and immune response that requires a computational and systems biology research approach. Here, we used a novel technology, CyTOF mass cytometry (CyTOF), to characterize the multi-cellular immune response to severe trauma. By characterizing a massive number of circulating immune cell markers at 1 day after severe trauma, we are able to construct, for the first time, an immune phenotype of trauma. In this pilot study, we focused on myeloid and lymphoid markers that comprise a subset of our comprehensive immunophenotyping assay.
Methods: Blood mononuclear cells from severe trauma patients with injury severity score >20 and from normal controls were collected within 24 hours of initial injury. Cells were stained with lymphoid- or myeloid-cell centric CyTOF staining panels consisting of a combination of 35 cell-surface and intracellular subset identifying marker antibodies each. CyTOF staining data was analyzed using multi-dimensional visualization and statistical methods to reveal trauma-associated changes in circulating immune cell subsets using normal blood cells for comparison.
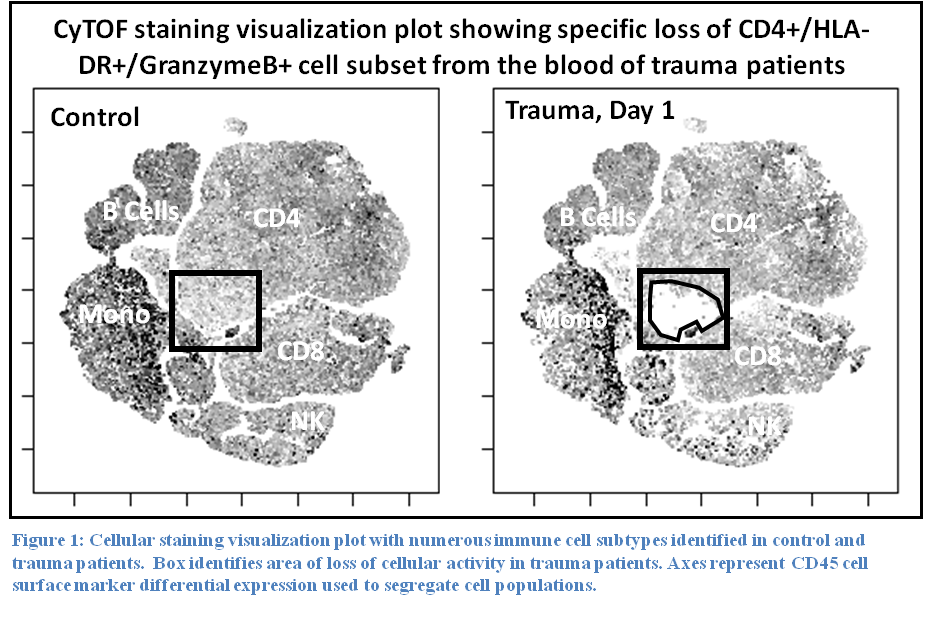
Results: Staining profiles of mononuclear cells using our lymphoid CyTOF panel revealed a specific loss of a unique subset of CD4 T cells that express MHC class II, GranzymeB, PD-1, and Perforin (Figure 1). Staining total leukocyte cells with a myeloid panel showed a complete loss of cells showing eosinophil/basophil staining phenotype and marked reduction in NK cells. As expected, neutrophils were dramatically increased in trauma patients. Other lymphoid populations were unchanged.
Conclusion: These preliminary results demonstrate the feasibility of CyTOF for large-scale profiling of circulating immune cells in trauma patients. Our pilot study of myeloid and lymphoid markers identified specific trauma-induced cellular phenotypic changes that are consistent with movement of certain immune cell types out the circulatory system and possibly towards affected tissues or organs.