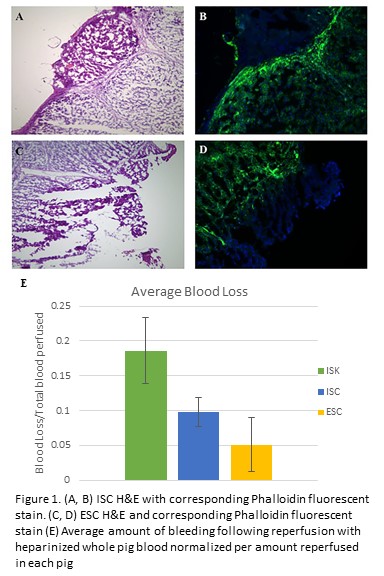
E. A. Fallon1, A. Rossi1, W. G. Cioffi1, A. Ayala1, D. S. Heffernan1 1Brown University / Rhode Island Hospital,Department Of Surgery,Providence, RI, USA
Introduction: Sepsis remains a leading cause of death, with much of the morbidity and mortality deriving from immune dysfunction. Specifically, sepsis induces profound immunoparalysis. We have shown a role for the checkpoint protein Programmed cell death receptor-1 (PD-1) in regulating the immune response to sepsis. Although considerable data has emerged regarding the role and function of PD-1 and its primary ligand PD-L1 upon lymphocytes, little information is available regarding the role of PD-1/PD-L1 upon neutrophils in response to intra-abdominal murine sepsis.
Methods: Cecal ligation and puncture (CLP) versus sham laparotomy was undertaken in 8 to 12 week old male C57BL/6 mice. At 24 hours, mice were euthanized and peritoneal cells were obtained by lavage. Samples were analyzed by flow cytometry with monoclonal antibodies. Neutrophils were identified as CD11b+/Ly6G+ cells. These cells were then further analyzed for PD-1 or PD-L1 expression. Chi-squared and Mann-Whitney-U tests were used for statistical analysis.
Results: Sepsis was noted to induce an increase in both absolute number of neutrophils into the peritoneal cavity, as well as neutrophils as a percentage of peritoneal cells (21% versus 43%; p<0.05). PD-1 expression upon peritoneal neutrophils was unchanged following sepsis (52% versus 59%; p=0.1), whereas PD-L1 expression was increased upon peritoneal neutrophils 22% vs 51%; p=0.02).
Conclusion: Neutrophils play a critical role in the immune response to polymicrobial sepsis. Although expression of PD-1, a key checkpoint protein of sepsis pathophysiology, upon peritoneal neutrophils is unchanged in response to sepsis, PD-L1 expression is elevated. This is akin to previous findings of the PD-1/PD-L1 relationship on lymphocytes. Our data support the concept that upregulation of PD-L1 is associated with migration into the peritoneal cavity in response to sepsis.