P. T. White1, C. Subramanian1, R. Kuai2, J. Moon2,5, B. M. Timmermann3, A. Schwendeman2, M. S. Cohen1,4 5University Of Michigan,Department Of Biomedical Engineering,Ann Arbor, MI, USA 1University Of Michigan,Department Of Surgery,Ann Arbor, MI, USA 2University Of Michigan,College Of Pharmacy,Ann Arbor, MI, USA 3University Of Kansas,Department Of Medicinal Chemistry,Lawrence, KS, USA 4University Of Michigan,Department Of Pharmacology,Ann Arbor, MI, USA
Introduction: Triple negative breast cancer (TNBC) remains a therapeutic challenge today, highlighting the critical need for discovery and development of safer novel therapies. Withanolides are a unique class of naturally-derived Hsp90 inhibitors that are highly efficacious in preclinical TNBC models, but fairly hydrophobic in vivo. Synthetic high-density lipoprotein (sHDL) nanoparticles have been safely used in clinical cardiology trials and recently shown by our group to conjugate to withanolides to improve drug delivery, solubility, and efficacy in aggressive adrenal cancers in vivo. sHDL provides targeted drug delivery as a ligand to the SR-B1 surface receptor leading to cholesterol uptake and efflux. TNBCs highly overexpress SR-B1 and we hypothesize that combining withanolides with sHDL will enhance their delivery to TNBCs through improved targeting via SR-B1.
Methods: Validated human TNBC cell lines (MD-MBA468LN,MDA-MB231,SUM159) were evaluated for SR-B1 mRNA expression levels by qPCR, and protein levels confirmed by Western blot. Fluorescent labeled sHDL was used to evaluate SR-B1 mediated drug uptake in vitro under fluorescence microscopy, and used for tumor targeting in vivo with whole body IVIS spectrum imaging of mouse xenograft tumors (MD-MBA468LN). Withanolide (WGA-TA) IC50 values were obtained using 72 h CellTiter-Glo (CTG) assays.
Results: All TNBC cell lines had significantly (p<0.01) higher SRB1 expression by qPCR and Western blot compared to fibroblast or Jurkat cells (SUM159 4-5 fold, MDA-MB468LN 8-10 fold, MDA-MB231 2-4 fold). Fluorescent labeled sHDL uptake in vitro demonstrated cytosolic uptake of nanoparticles at significantly (p<0.05) higher levels within the high SR-B1 expressing cell line (MDA-MB468LN) compared to the lower SR-B1 expressing cell line (MDA-MB231). Fluorescent sHDL uptake was almost completely inhibited by receptor saturation through pre-treatment with 10-fold excess sHDL (p<0.05). In vivo tumor uptake of fluorescent labeled sHDL using IVIS spectrum imaging in the MDA-MB469LN mouse xenograft model demonstrated highest radiant efficiency in the tumor, with uptake in the liver where it is metabolized and significantly (p<0.05) lower radiant efficiency in other organs. Using CTG assays, treatment with sHDL up to 20 μM showed no changes to cell viability. In TNBC, IC50 values of WGA-TA vs. sHDL-WGA-TA were not statistically different with IC50 concentrations ranging from 18-125 nM. Both formulations had significantly lower IC50 values when compared to MCR5 control cells (6-41 fold lower; p<0.05).
Conclusion: Conjugation of WGA-TA with sHDL nanoparticles confers improved SR-B1 receptor-mediated targeting both in vitro and in vivo in TNBC without inhibiting the potency of withanolides in these tumor cells. This nanocarrier delivery system warrants further translational evaluation in vivo in patient-derived xenograft of TNBC to determine if improved targeting will confer enhanced treatment benefit and survival.
P. Patel1, T. Kato1, H. Ujiie1, D. Lee1, J. Ahn1, H. Hu1, J. Zheng2,3, K. Yasufuku1,2,3 1University of Toronto,Division Of Thoracic Surgery,Toronto, Ontario, Canada 2University Health Network,TECHNA Institute,Toronto, Ontario, Canada 3University of Toronto,Institute Of Biomaterials & Biomedical Engineering,Toronto, Ontario, Canada
Introduction:
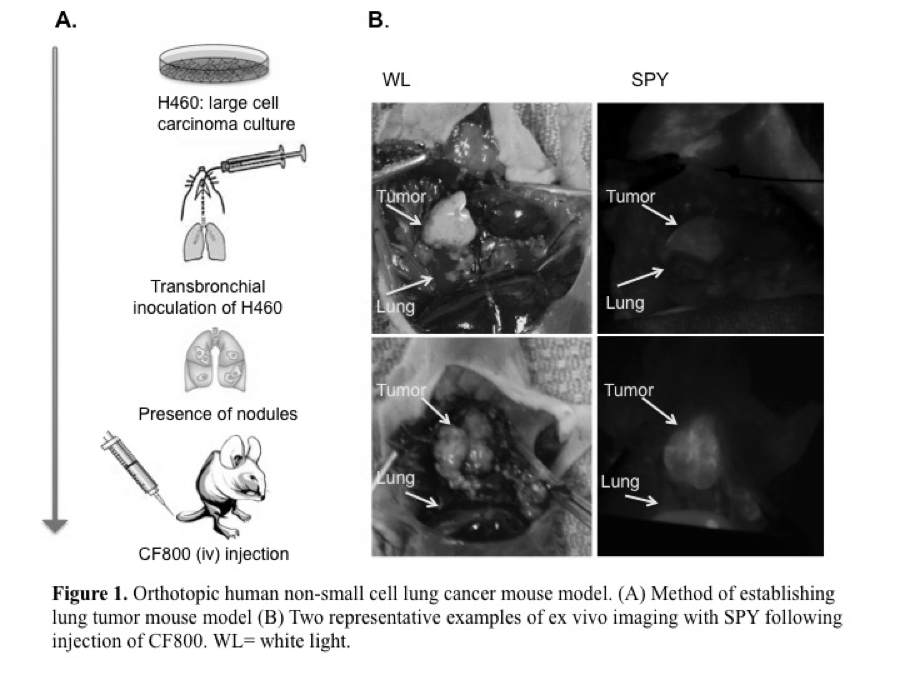
To investigate the feasibility of CF800, a novel PEGylated nano-liposomal imaging agent containing indocyanine green (ICG) and iohexol, for real-time near infrared (NIR) fluorescence and computed tomography (CT) image-guided surgery in an orthotopic lung cancer model in nude mice.
Methods:
CF800 was intravenously administered into 13 mice bearing the H460 orthotopic human non-small cell lung cancer. At 48 h post-injection (peak liposome accumulation time), ex vivo NIR and CT imaging was performed. A clinical NIR imaging system (SPY®, Novadaq) was used to measure the fluorescence intensity of tumor and adjacent lung tissue. Tumor-to-background-ratios (TBR) were calculated in inflated and deflated states. The mean Hounsfield unit (HU) of lung tumor was quantified using the CT data set and a semi-automated threshold-based method. Histological evaluation was performed using Hematoxylin & Eosin, and immunofluorescence macrophage marker F4/80 and endothelial cell marker CD31, and compared to the liposomal fluorescence signal obtained from adjacent tissue sections.
Results:
The fluorescence TBR measured when the lung is in the inflated state (2.0 ± 0.58) was significantly greater than in the deflated state (1.42 ± 0.38) (p<0.05). Mean fluorescence signal in tumor was highly variable across samples, (49.0 ± 18.8 AU). CT image analysis revealed greater contrast enhancement in lung tumors (a mean increase of 110 ± 57 HU) when CF800 is administered compared to the non-contrast enhanced tumors (p <0.05).
Conclusion:
This preliminary data suggests that the high fluorescence TBR demonstrated with the SPY system, and CT tumor contrast enhancement provided by CF800 may have clinical utility in aiding delineation and localization of lung cancer during CT and NIR image-guided surgery.
R. Jaskula Sztul1,3, G. Chen2, A. Harrison1, S. Gong2, H. Chen1,3 1University Of Wisconsin,Surgery,Madison, WI, USA 2University Of Wisconsin,Wisconsin Institutes For Discovery,Madison, WI, USA 3University Of Alabama,Surgery,Birmingham, Alabama, USA
Introduction: Although neuroendocrine tumors (NETs) are slow growing, they are frequently metastatic at the time of their discovery and no longer amenable to curative surgery. Therefore, there is a great need to develop novel therapeutic strategies both to reduce tumor burden and control the release of hormones. To address this need, we developed and optimized a family of novel multifunctional upconversion nanoparticle (UCNP)-based theranostic unimolecular micelles capable of delivering a newly reported anticancer drug AB3 for NET-targeted combination chemotherapy, photodynamic therapy (PDT), and bioimaging. These UCNP-based micelles conjugated with photosensitizer (Rose Bengal, RB) specifically target NET cells using somatostatin analog (KE108). In the current study we assessed the antitumor effects of the nanotheranostic micelles both in vitro and in vivo.
Methods: Stable UCNP-based micelles were prepared in an aqueous solution using multi-arm star amphiphilic block copolymer. KE108 was conjugated for active tumor-targeting. AB3 was loaded into the photosensitive hydrophobic core of the resulting micelles. The effect of 980nm on singlet oxygen generation and in vitro drug release of the UCNP-based micelles were studied. Cell proliferation was assessed by MTT assay in human medullary thyroid cancer cell line (MZ-CRC-1) treated with a family of AB3-loaded micelles (AB3 mM) for 48h with or without 980nm irradiation. The effect of the KE108 targeting ligands on the cellular uptake of the nanotheranostic micelles was measured by flow cytometry and confocal laser scanning microscope (CLSM). The antitumor efficacy of AB3-loaded micelles was determined in GI neuroendocrine (BON) xenografts after two intravenous injections performed with 7 day interval with a dose of 20 mg/kgBW. The group treated with UCNP-based micelles containing RB was irradiated for 15 min with 980nm laser at 4h post injection.
Results: The family of UCNP-based NET-targeting unimolecular micelles was developed for targeted delivery of AB3 and RB to NETs. UCNP-based micelles for targeted and combined chemotherapy with PDT exhibited the strongest antiproliferative effect. Moreover, the targeted micelles exhibited a much higher cellular uptake than non-targeted micelles based on flow cytometry and CLSM analyses. Additionally, AB3 loaded micelles conjugated with RB and KE108 (i.e., T-RB-AB3), enabling combined chemo-therapy and PDT, induced the best antitumor efficacy (82% reduction in tumor volume) and did not cause any significant changes in body weight or survival. Pathological assessment of H&E-stained sections of different organs of mice treated with T-RB-AB3 micelles did not indicate any signs of inflammation or necrotic regions.
Conclusion:The AB3-loaded UCNP-based micelles conjugated with both RB and KE108, offering combination chemotherapy and PDT, are more effective at suppressing NET cell growth while having minimal toxicity to non-NET cells.
M. M. Hodges1, C. Zgheib1, J. Hu1, J. Xu1, K. W. Liechty1 1University Of Colorado Denver,Laboratory For Fetal And Regenerative Biology, Department Of Surgery,Aurora, CO, USA
Introduction: In 2014, the United States Center for Disease Control and Prevention (CDC) estimated that 29.1 million Americans (9.3% of Americans) were living with diabetes, with the economic burden of diabetes related wound care estimated as $38.6 billion in 2007. With the global prevalence of diabetes expected to double between 2000 and 2030, there will be an increasing need for innovative technologies to address the commensurate rise in chronic wounds. We have previously demonstrated that diabetic wounds exhibit markedly decreased levels of the anti-inflammatory microRNA miR-146a. Furthermore, we have shown improved healing of diabetic wounds after treatment with a lentiviral construct expressing stromal derived growth factor (SDF-1α). We hypothesize that the improved healing observed in diabetic wounds after SDF-1α treatment is partly due to increased miR-146a expression and the subsequent decrease in inflammation.
Methods: To test our hypothesis we isolated dermal fibroblasts from the skin of 10 week old diabetic (Db/Db) and non-diabetic (Db/+) mice. These fibroblasts were then cultured and transfected with a lentivirus expressing either SDF-1α or green fluorescent protein (GFP) as a control. RNA was extracted from these fibroblasts, and real time PCR analysis was utilized to quantify the gene expression of NFkB, TRAF6, IRAK1, IL-6, MIP2 (IL-8), and miR-146a.
Results: When compared to fibroblasts from the skin of non-diabetic mice, fibroblasts isolated from the skin of diabetic mice demonstrated significantly decreased levels of miR-146a and increased levels of NFkB, TRAF6, IRAK1, IL-6, and MIP2 (IL-8). When transfected with a lentivirus expressing SDF-1α, fibroblasts from the skin of diabetic mice demonstrated significantly increased expression of miR-146a, and significantly decreased expression of NFkB, TRAF6, IRAK1, IL-6, and MIP2 (IL-8).
Conclusions: The significantly increased expression of inflammatory mediators NFkB, TRAF6, IRAK1, IL-6, and MIP2 (IL-8) observed in diabetic fibroblasts is nearly reversed with lenti-SDF-1α transfection. Similarly, the decreased level of miR-146a observed in diabetic fibroblasts is completely reversed when diabetic fibroblasts are transfected with lenti-SDF-1α. The accelerated wound healing previously observed in diabetic skin after lenti-SDF-1α treatment may be due in part to the increased expression of miR-146a and decreased expression of inflammatory mediators NFkB, TRAF6, IRAK1, IL-6, and MIP2 (IL-8). These results support further investigation of the role of SDF-1α in the pathogenesis of diabetic wound healing impairment and as a possible therapeutic target in the treatment of diabetic wounds.
I. J. Lawandy1, B. A. Potz1, N. Y. Elmadhun1, A. D. Lassaletta1, J. A. Feng1, F. W. Sellke1 1Brown University,Surgery/Cardiothoracic Surgery/ Warren Alpert Medical School,Providence, RHODE ISLAND, USA
Introduction: Autophagy serves as a cellular protective mechanism against alcohol induced tissue injury but can also be detrimental leading to apoptosis. Our lab has previously shown moderate alcohol consumption in a swine model of metabolic syndrome worsens glucose metabolism by altering activation of the insulin signaling pathway in the liver. We examined the effect of alcohol consumption on apoptosis and autophagy signaling in the liver in our clinically relevant animal model of chronic myocardial ischemia and hypercholesterolemia.
Methods: Twenty-six Yorkshire swine were fed a hypercaloric, high-fat diet for 4 weeks then split into 3 groups: hypercholesterolemic diet alone (HCC, n= 9), hypercholesterolemic diet with vodka (HCV, n= 9), and hypercholesterolemic diet with wine (HCW,n = 8) for 7 weeks. Animals underwent euthanasia and liver tissue samples were harvested for analysis.
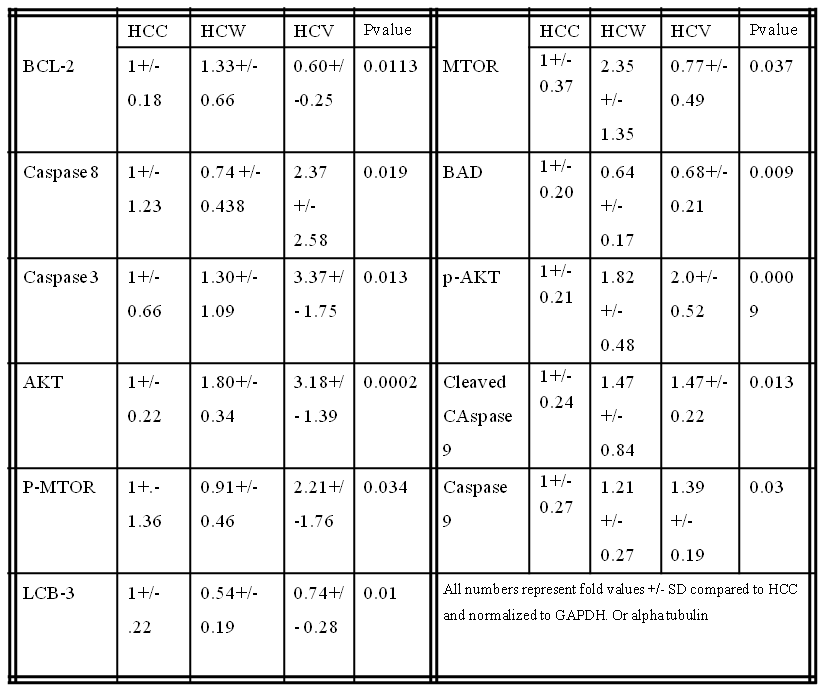
Results: There were significant increases in the pro-apoptotic proteins caspase 3, caspase 8, caspase 9 and cleaved caspase 9 in the HCV group and a trend increase in the HCW group compared to control. There was a significant decrease in pro-apoptotic protein Bad in the HCW and HCV groups compared to control. There was a significant increase in anti-apoptoic signal BCL-2 in the HCW group compared to the HCV group and trend increase in the HCW group compared to control. There were significant increases in pro-survival proteins AKT and p-AKT in the HCW and the HCV group compared to control. There was significant increase in MTOR in HCW compared to HCV and a trend increase in compared to control. There was a significant increase in p-MTOR in the HCV group compared to the control. There was a significant decrease in autophagy protein LCB-3 in the HCW and a trend decrease in HCV compared to the control. (See table)
There was a trend increase in anti-apoptotic signal p-BCL-2 in the HCW and HCV groups (p=0.151) compared to control. There were trend increases in the HCV group in AMPKA (p= 0.071) and TNF-alpha (p=0.071) compared to control. There was a trend increase pro- autophagy protein Beclin 1 in the HCW group (p=0.431) compared to control. There were trend increases in pro-autophagy proteins Lamp-1 and Lamp-2 in the HCW group and a trend decrease in the HCV group (p=0.120) compared to control. There were no significant differences in cleaved Caspase 3 (p=0.296), Bax (p= 0.169), p-Bad (p =0.675), LKB-1 (p=0.523), P-AMPKA (p=0.758) or ATG5 (p=0.319) compared to the control.
Conclusions: Moderate Alcohol consumption altered cell survival protein expression related to apoptosis and autophage in pig liver in the setting of hypercholesterolemia. Vodka may induce more pro apoptotic pathways and wine may induce more pro survival pathways in pig liver tissue.
K. Inomata1, H. Yagi1, K. Tajima1, H. Shimoda1, T. Hibi1, Y. Abe1, M. Kitago1, M. Shinoda1, O. Itano1, Y. Kitagawa1 1Keio Universiry,Surgery,Shinanomachi, Shinjukuku, TOKYO, Japan
Introduction: Recently, regenerative medicine approaches have been made great advantage, therefore evaluation of its feasibility in animal model is absolutely necessary for clinical application. However, pre-clinical model for the study of liver regeneration is yet to be established. Regeneration process of the liver requires continuous regenerative signals induced by hepatic injury, on the other hand, transplanted cells require functional advantage compared to the remnant cells to repopulate efficiently in recipient’s body. One of the sufficient models for these demands is clearly retrorsine with partial heaptectomy (PH) model. However, no study has been reported using large animal including porcine. Here we demonstrate the first established porcine model with retrorsine and PH.
Methods: Göttingen miniature pigs were treated with 2 intra-peritoneal administrations of retrorsine in different doses; 30mg, 50mg and 100mg/kg respectively, or saline as a control, 2 weeks apart. Four weeks after the second administration of retrorsine, animals underwent PH in different volumes; 50%, 60% and 85%. The animals were sacrificed on 10, 14 and 28 days after the PH and the resected liver volume and histological alterations were evaluated. Blood samples were obtained every 7 days until and every 3 days after the PH to analyze hepatobilially enzymes and synthesized proteins. The quantity of serum and liver tissue retrorsine concentration was determined by LC-MS/MS. Hematoxylin and eosin staining and immunohistochemical staining for Ki-67 and EpCAM were performed on the liver tissue.
Results: The Animals injected 100 mg/kg retrorsine or performed 85% PH were resulted in dead with severe liver injury. Blood test demonstrate that distinct liver disorder was sustained after PH. Liver regeneration was inhibited in animals as 20% volume reduction at day 10 of 60% PH animals with pre-surgical administration of 50mg/kg retrorsine. The histological study demonstrated the explosive puff and cytoplasmic vacuoles were remained in retrorsine treated animals at day 10. The immunohistochemical staining showed the expression of Ki-67 in hepatocytes and EpCAM in biliary epithelium were suppressed in retrorsine treated animals. LC-MS/MS determined the quantity of accumulated retrorsine in the liver tissue while retrorsine was not detected in the blood.
Conclusion: We could successfully demonstrate the first large animal model of retrorsine and 60% PH characterized by sustained liver injury with suppression of hepatic regeneration. We believe that it is a promising model for improving preclinical study of liver regenerative medicine.
H. Aoki1,2, M. Aoki1,2, E. Katsuta1,2, J. Yong3, X. Wang3, H. Zhou3, S. Spiegel2, K. Takabe1,2 1Virginia Commonwealth University,Division Of Surgical Oncology, Department Of Surgery,Richmond, VA, USA 2Virginia Commonwealth University,Department Of Biochemistry & Molecular Biology,Richmond, VA, USA 3Virginia Commonwealth University,Department Of Microbiology And Immunology,Richmond, VA, USA
Introduction: Obstructive jaundice is one of the classic signs of pancreatic head mass, or tumor in the biliary tree. Although there are numerous publications on clinical management of obstructive jaundice, there are very few reports regarding the effects of it on cancer biology itself partly due to lack of stable model. The standard murine model for cholestasis is the partial ligation of the common bile duct (pBDL), which is known to have very short survival, usually less than 3-4 days. Recently, a long term survival cholestasis model that totally ligate hepatic bile duct (tHBDL) was reported. Here we compared tHBDL model with standard pBDL model on their characteristics.
Methods: C57Black6 mice were subjected to sham, pBDL and tHBDL operations. For the tHBDL model, total ligation of the hepatic bile duct was performed at the level where left and median lobe bile ducts, as well as the duct from upper right lobe were completely obstructed, whereas right lower lobe bile duct was spared. Of note, cholecystectomy was added to all tHBDL model. For pBDL model, the suture was tied down tightly at the common bile duct with surgical needle placed by the side, and then the needle was removed leaving a defined lumen within the ligation. Survival was determined by humane endpoints and assessed by Kaplan-Meier method. Weights of body, liver and spleen were measured on the indicated days. Intraoperative complications were also recorded.
Results: 24 mice that received pBDL mice died between 3-4 days after operation. On the other hand, mice that underwent tHBDL were observed to survive over 14 days, longest survivor was over 2 months. After 2 days, pBDL model showed the significant body weight loss compared with sham and tHBDL. An increase of liver weight was observed in both pBDL and tHBDL groups, where it was much heavier in tHBDL group than pBDL group. Spleen of tHBDL model was heavier than other groups, on the other hand, weight of spleen in pBDL group was lighter than sham surgery group. In tHBDL model, enlargement of right lobe was observed. Most common complication during the procedure was bleeding from portal vein or liver and technique for tHBDL model was stabilized due to good exposure of procedure field with assistance.
Conclusion: Total hepatic bile duct ligation model was clearly more stable model than standard partial bile duct ligation model, which is most commonly used. Given the longer survival, total hepatic bile duct ligation model provide us tool to investigate the biological impact of obstructive jaundice.
V. X. Zhou1, M. Lolas1, T. T. Chang1 1University Of California – San Francisco,Surgery,San Francisco, CA, USA
Introduction: Liver transplantation is currently the only treatment for end-stage liver disease (ESLD) and is limited by the critical shortage of donor organs. Implantation of liver organoids generated ex vivo by three-dimensional (3D) culture techniques may serve as an adjunct or alternative to liver transplantation. Efficient high-engraftment methods of introducing organoids into the liver parenchyma have not been established. In this study, we aim to develop a reliable surgical technique to implant liver organoids into the liver and assess their ability to engraft and function.
Methods: Liver organoids composed of either hepatocytes alone or hepatocytes co-cultured with stellate cells were generated by 3D culture in rotating wall vessel bioreactors. Primary hepatocytes and stellate cells were isolated from ROSA26 C57BL/6 mice, in which β-galactosidase was expressed under a ubiquitous promoter, so that engrafted cells could be easily identified by X-gal staining when implanted into wild-type mice. Some recipient mice underwent simultaneous two-thirds partial hepatectomy to determine whether hepatocytes within implanted organoids proliferated in response to acute liver insufficiency. As control, freshly isolated primary hepatocytes were transplanted as single-cell suspensions. At various time-points after implantation, engraftment of liver organoids was qualitatively and quantitatively determined.
Results: Confocal microscopy analysis demonstrated that hepatocytes within liver organoids had cortical actin organization and produced extracellular matrix. Organoids maintained their 3D structure up to 7 days after implantation within recipient livers and engraftment efficiency was superior to that of single cells. Generating liver organoids with stellate cell co-culture and performing two-thirds partial hepatectomy on recipient mice did not appear to improve engraftment efficiency.
Conclusion: Implantation of liver organoids generated by 3D tissue culture is promising for development as a novel surgical therapy for ESLD. Further optimization of 3D culture and implantation techniques are required to increase engraftment efficiency.
F. J. Bohanon1, X. Wang1, O. A. Nunez Lopez1, A. Kandathiparampil1, N. Ye1, S. J. Vasudevan1, H. Chen1, J. Zhou1, R. S. Radhakrishnan1 1University Of Texas Medical Branch,Galveston, TX, USA
Introduction: STAT3 plays an important role in many physiologic and pathologic processes by regulating cell proliferation, differentiation, and metabolism. It has been reported that the STAT3 pathway is involved in hepatic fibrogenesis. Activated hepatic stellate cells (HSC) are the major effector cells for liver fibrosis, and HSC activation is characterized by increased proliferation and over-expression of α-smooth muscle actin, and extracellular matrix (ECM) proteins. Recently, our team developed a series of novel small-molecule inhibitors of STAT3 and demonstrated their potent anti-tumor effects in breast cancer cells. In the present study, we tested their anti-fibrogenic effects using activated HSC.
Methods: The proliferation of activated human and rat HSC cell lines LX-2 and HSC-T6 were measured by Alamar Blue. Cellular proteins were determined by Western blots and immunofluorescence. Flow cytometry was conducted for cell cycle study. Yo-pro-1 staining was used for apoptosis.
Results:HJC0123 treatment significantly inhibited LX-2 and HSC-T6 cell growth and attenuated HSC activation marker α-smooth muscle actin expression. The expression of endogenous ECM collagen type I and fibronectin was suppressed by HJC0123 in a dose- and time-dependent manner. TGFβ has been identified as the most potent stimulator for ECM. Our data show that in LX-2 cells, TGFβ significantly up-regulated collagen type I and fibronectin expression, and pretreatment with HJC0123 prevented TGFβ-stimulated ECM production. Furthermore, pretreatment with HJC0123 impaired TGFβ-induced pSmad2/3 expression and nuclear translocation, indicating that the TGFβ/pSmad pathway plays a role in ECM regulation by HJC0123.
Conclusion:HJC0123 inhibits HSC proliferation, suppresses endogenous and TGFβ-induced ECMs expression. HJC0123 is a promising anti-hepatic fibrogenic agent.
J. Carr1, S. King1, C. Dekaney1 1University Of North Carolina At Chapel Hill,Chapel Hill, NC, USA
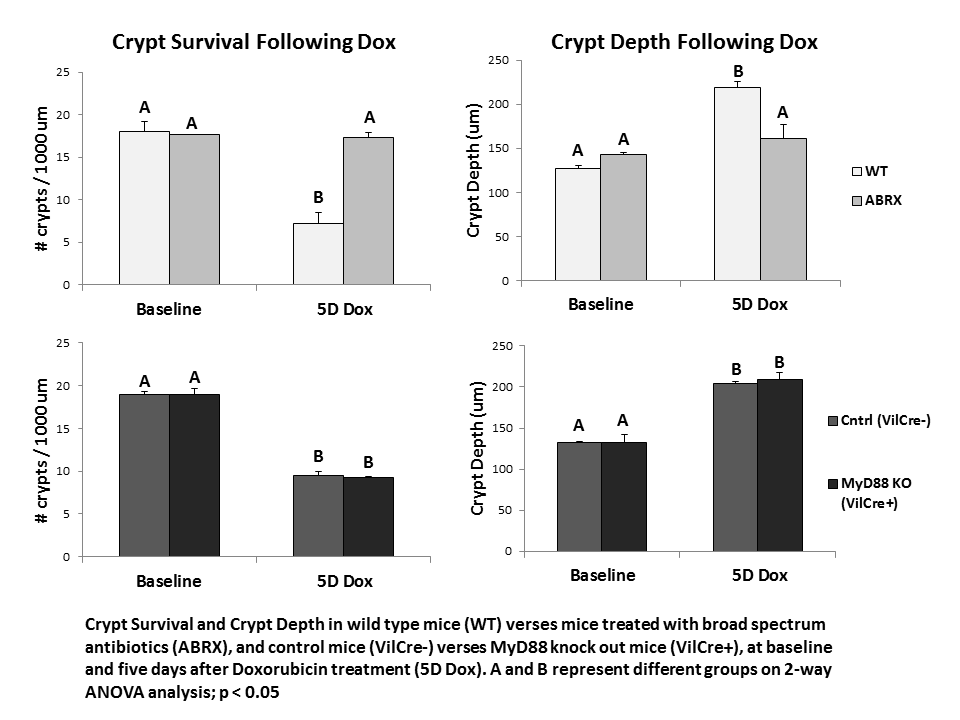
Introduction: Enteritis is a significant side effect of chemotherapy, and is a potential dose-limiting complication. Pharmacotherapy can diminish symptoms, but there are no effective therapies to prevent or treat the intestinal epithelial injury associated with enteritis, most likely because the mechanisms guiding epithelial damage and repair are unknown. Our preliminary studies suggest that enteric bacteria play a critical role in precipitating mucosal damage after treatment with doxorubicin (Dox), a common chemotherapeutic drug. Following Dox, mice treated with broad-spectrum antibiotics (ABRX) do not show the small intestine crypt loss, increased crypt depth, or alterations in proliferation demonstrated in wild type mice (WT). Bacteria and bacterial products signal via distinct toll like receptors (TLRs), while MyD88 is a key adapter molecule utilized by nearly all TLRs to activate downstream signaling pathways. ‘Knocking out’ MyD88 in the intestinal epithelial cell (IEC) specifically eliminates IEC bacterial signaling, and therefore allows an evaluation of the role of IEC bacterial signaling in mediating the bacterial contribution to enteritis. In the current study, we utilize this knock out model to determine whether elimination of bacterial IEC signaling is responsible for protection from Dox-induced enteritis.
Methods: At 10 weeks of age, control or IEC-specific MyD88 KO mice (VillinCre/MyD88fl/fl) were treated with a single intraperitoneal dose of Dox (20 mg/kg) and euthanized five days following treatment. The jejunum was fixed and stained with H&E for histologic analysis.
Results: While ABRX mice demonstrate significantly less weight loss than WT mice five days after Dox, MyD88 KO mice demonstrated weight loss equivalent to WT mice (WT 19.5% ± 2.4; ABRX 5.1% ± 4.6; MyD88 KO 17.5% ± 3.0). ABRX mice demonstrate significantly greater crypt survival than WT mice five days after Dox, and do not demonstrate increased crypt depth typical of WT mice in their hyper-proliferative state after Dox, while MyD88 KO mice demonstrate a phenotype comparable to WT mice (graph).
Conclusions: In response to insult with the chemotherapeutic Dox, MyD88 KO mice demonstrate intestinal damage followed by hyper-proliferation similar to WT mice. This suggests that the protection from damage demonstrated in ABRX mice is not due to elimination of epithelial bacterial signaling. Future studies will investigate other potential signaling pathways (e.g. penetration of a compromised epithelial barrier) to elucidate how depletion of bacteria protects from Dox-induced damage. This will allow determination of a prospective molecular target for prevention of chemotherapy-induced enteritis.
A. L. Franklin1, T. Iordanskaia1, M. Barberio1, D. Pillai1, M. Hubal1, E. P. Nadler1 1Children’s National Health System,General Surgery,Washington, DC, USA
Introduction:
The pathogenesis of non-alcoholic fatty liver disease (NAFLD) is associated with obesity and insulin resistance. Hispanics have the highest prevalence compared to non-Hispanic Whites and Blacks, with Black patients having the lowest prevalence. Differences in the incidence of NAFLD among race/ethnicity may be related to visceral adipose tissue (VAT), as Blacks have less VAT than Whites and Hispanics. The exact mechanisms behind the ethnic and racial discrepancies are unclear. Exosomes are cell-derived vesicles that contain messenger RNA, microRNA, and proteins, that are potential mediators of systemic disease via the delivery of genetic material to distant organs. We believe that adipocyte-derived exosomes may be the direct mechanistic link between obesity and NAFLD, and exosomal differences may explain the ethnic and racial disparities. We have previously shown that adipocyte exosomes from mixed race/ethnicity donors lead to a dysregulation of gene expression of transforming growth factor beta-1 (TGFΒ-1) mediators in cultured hepatocytes. We sought to determine if ethnic differences among adipocyte exosomes exist.
Methods:
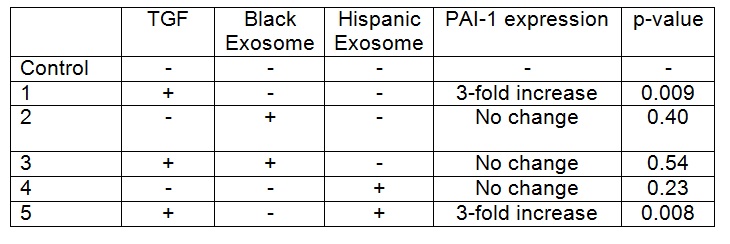
Institutional Review Board approval and patient or parental/guardian consent was obtained. HepG2 cells were grown to 60-70% confluence and then co-cultured with TGFΒ-1 for 48 hours. VAT exosomes from one Black (BMI 51) and one Hispanic (BMI 46) female patient were applied to HepG2 cells in culture with and without TGFΒ-1 exposure. PAI-1 protein expression was analyzed with ELISA, and compared via t-test. Experiments were performed in duplicate and repeated once for each donor.
Results:
TGFB-1 exposure to HepG2 cells increased PAI-1 expression when compared to control cells (p= <0.05). Addition of exosomes from the Black patient decreased PAI-1 expression in HepG2 cells exposed to TGFB-1 (p=0.034), with expression levels that were similar to baseline (Table). In contrast, there was no difference in PAI-1 expression in HepG2 cells exposed to TGFB-1 and exosomes from the Hispanic patient when compared to cells co-cultured with TGFB-1 alone. Exosomes from neither donor altered PAI-1 expression in the absence of TGFB (p= >0.05).
Conclusion:
HepG2 cells co-cultured with TGFΒ-1 had an increase in PAI-1 protein expression. VAT exosomes from a Black patient inhibited TGFB-1 induced production of PAI-1 in hepatocytes. However, exosomes from a Hispanic patient did not inhibit this TGFB-1 induced upregulation of PAI-1. Racial differences in exosomal content and function likely exist, and may be the mechanism behind the known ethnic and racial differences in NAFLD prevalence.
R. Patel1, S. Misra1, S. Joseph1 1Texas Tech University Health Sciences,Department Of Surgery,Odessa, TX, USA
Introduction:
Acute appendicitis can present with a wide range of symptoms from an indolent course to severe peritonitis and overwhelming sepsis. The patients clinical status often indicates the severity of the disease and if perforation and diffuse peritonitis is present. While there have been many attempts to identify patients who may develop sepsis and peritonitis, little has been found as to the cause of the most severe cases.
Actinomyces, a gram positive facultative anaerobe, is part of the normal flora of the mouth, GI tract, and female vagina, however when pathologic it causes severe inflammation, gangrene, perforation, and obstruction. Actinomyces can easily be identified on pathologic examination by the formation of sulfur granules. We hypothesized that Actinomyces could be a causative agent in patients who present with perforated or gangrenous appendicitis.
Methods:
We did a retrospective review of the last 102 appendix specimens removed for acute appendicitis at our institution. Pathologic specimens were reviewed by a single pathologist to identify sulfur granules or actinomyces species within the specimen. Demographic data, lab value, and radiology were reviewed for all patients in the review. Appendiceal specimens were categorized as either gangrenous/perforated or acute inflammation.
Results:
There were a total of 20 cases of perforated/gangrenous appendicitis amongst our 102 cases (19.6%). For patients with gangrene/perforation (n=20) there were 7 cases of Actinomyces identified (35%). For the 82 patients with acute inflammation, Actinomyces was identified in 6 specimens (7%). p=0.0008
The average WBC level for all patients in our sample was 14.5. No clinically significant difference was found in WBC levels between gangrenous/perforated and acutely inflamed (14.4/ 14.5). However, patients with Actinomyces had a slightly higher average WBC of 15.3.
Conclusion:
In patients with gangrenous/perforated appendicitis there is a 35% chance that Actinomyces is present in the appendix. While in patients without perforation/gangrene there is a 7% chance of identifying Actinomyces. Patients with Actinomyces were found to have a slightly higher WBC level then the patients without, however this was not statically significant.
This data suggests that Actinomyces may be a cause of gangrenous/perforated appendicitis. Since Actinomyces is best treated with a long course of Penicillin based antibiotics, patients with gangrene/perforation may benefit from penicillin based treatment for longer than the standard 2 week course. Finally, Actinomyces may account for failure of non-operative management in selected patients.
J. Sell1, A. Oliviera1, E. Jensen1, E. Greeno1, M. Yamamoto1, J. Davydova1 1University Of Minnesota,Department Of Surgery,Minneapolis, MN, USA
Introduction: Interferon-α (IFN) is a cytokine known to have direct and indirect antitumor effects. Previous studies have shown that IFN-based chemoradiation therapy (CRT) can improve survival after resection of pancreas cancer. However, its clinical utility to this point has been limited due to the severe toxicity related to its use. Our aim in this study is to evaluate our group’s novel oncolytic adenovirus (OAd) which allows targeting IFN treatment to cancer cells while sparing healthy tissue. We have previously shown the ability of IFN to sensitize cancer cells to chemotherapy as well as demonstrated an increased therapeutic effect of the drug in immunocompetent models. This study was conducted to analyze the combination of 5FU chemotherapy and our OAd in vitro in order to assess the interaction of treatments and determine the optimum combined treatment regimen.
Methods: Treatments were analyzed in two pancreatic cancer and one esophageal adenocarcinoma cell lines: Panc1, S2013, and OE19. Recombinant OAds expressing luciferase rather than IFN were used to isolate the combination of 5FU and the virus. Two viral models were evaluated. Our therapeutic virus (Cox2) selectively replicative in Cox2 expressing cancer cells and a nearly identical but universally replicative virus (wild type) were compared. Cells were treated with 0, 1 or 10 viral particles per cell and 0, 5, 10, or 20 uM 5FU. Three timing regimens were used: simultaneous administration, 5FU 48 hours before virus, and virus 48 hours before 5FU. Crystal Violet and MTS Assays were used to measure cell death. Viral Copy number to assess viral replication was measured using qPCR.
Results: Cell death analysis showed time dependent killing of each virus, with a 2 day delay for the Cox2 virus. 5FU and each virus produced dose dependent cell death independently. There was a nearly full additive effect seen in cell death from combining treatments only with simultaneously given 5uM 5FU and virus. 10 and 20 uM concentrations and virus produced less than fully additive cell death. There was little additive effect seen in treatments of virus 48 hours before 5FU, across all concentrations. Patterns observed were similar for both S2013 and OE19. Studies with Panc1 are in progress. Viral copy experiments are in progress. Treatments with 5FU 48 hours before virus are in progress.
Conclusion: Our Cox2 OAd shows a killing effect similar to wild type in multiple cancer cell lines. When combined with 5FU treatment the expected addition in overall cell death from the independent treatments diminishes, more so as 5FU concentration increases. This may suggest an inhibition of the virus by 5FU. The killing ability and interaction of the combination treatments appears different when the timing of treatments is varied, suggesting the possibility of a treatment regimen with optimal therapeutic effect. Further studies investigating different chemotherapeutic drugs should be conducted to examine these trends.
M. Barneck2,3, M. De La Presa1, A. Poursaid1, M. Nourian1, M. A. Firpo1, J. T. Langell1,2 1University Of Utah,School Of Medicine, Department Of Surgery,Salt Lake City, UT, USA 2University Of Utah,Engineering,Salt Lake City, UT, USA 3Oregon Health And Science University,School Of Medicine,Portland, OR, USA
Introduction: Hospital Acquired infections (HAIs) are the most frequent adverse event in healthcare delivery worldwide, occuring in 5 -10% of acute care hospital admissions, costing the healthcare system an estimated $28-$45 billion annually. Increasing rates of antimicrobial resistant bacteria have increased the morbidity, mortality and cost of treating these infections.
Visible light sterilization (VLS) using high intensity LEDs and lasers have been adapted into several medical applications and have shown bactericidal activity. The inactivation mechanism is believed to be oxygen dependent photoexcitation of bacterial endogenous porphyrins, which leads to the production of a cytotoxic singlet oxygen species. While some research has been done in this field, an in depth comparison of common clinically pathogenic bacteria in a single study has not been published. Here we demonstrate a comprehensive evaluation of common gram-positive and gram-negative species at varying treatment times, intensities, bacterial concentrations, and photokinetic doses.
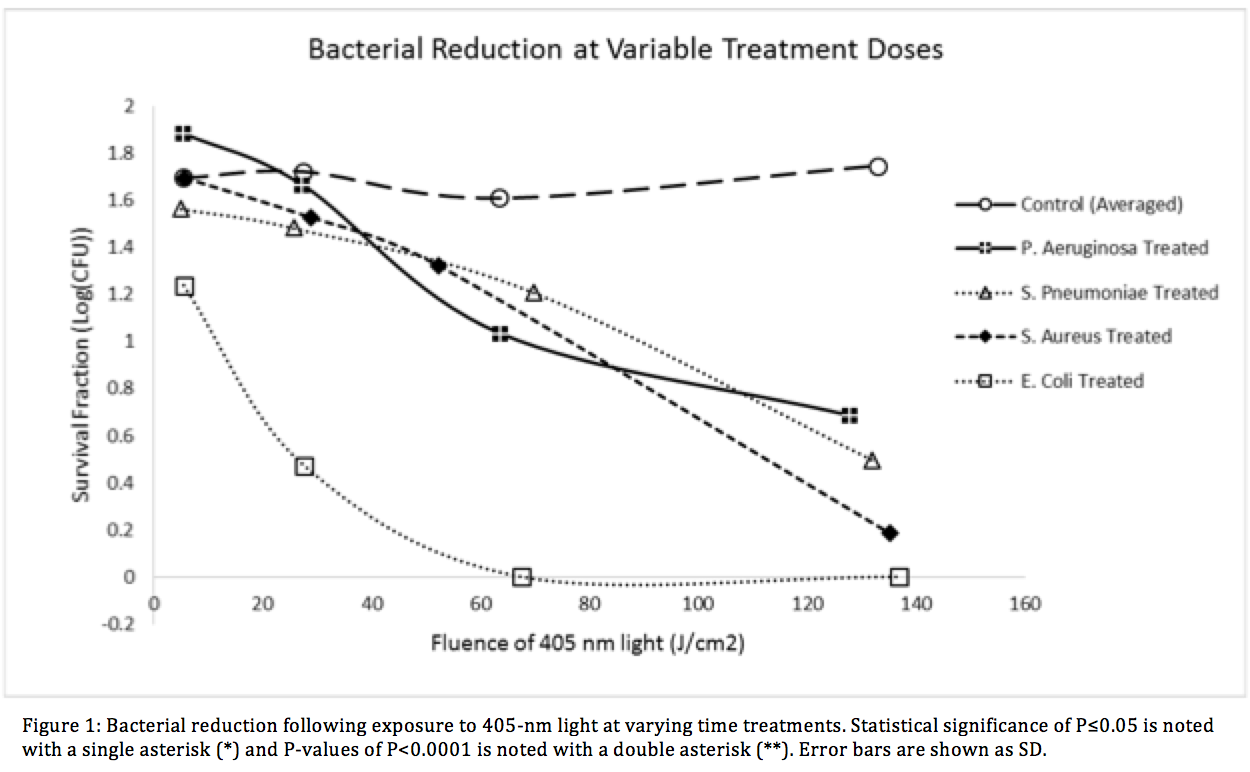
Methods: Visible light emitting diodes centered at 403.0-nm ± 10.8 were used to illuminate a panel of four relevant HAI facultative anaerobic bacteria, Escherichia coli, Staphylococcus Aureus (ATCC 29213), Pseudomonas Aeruginosa (ATCC 27853), and Streptococcus Pneumoniae (ATCC 49619). The E. coli K-12 strain transformed with the pCIG mammalian expression vector conferring ampicillin resistance via expression of the β-lactamase gene. Each bacteria was tested against the VLS with varying treatment durations, light intensities and colony forming unit (CFU) density. Treatment times ranged from 10 minutes to 16 hours, irradiance levels between 2.4mW/cm2 ± 0.3 and 8.9mW/cm2 ± 0.6, and fluence levels up to 132.98 ± 6.69 J/cm2.
Results: Dose dependent effects were visible in visible in all species. The largest inactivation occurred in β -lactam resistant E. coli at 6.27 ± 0.54 log for 250 minutes. Statistically significant results (<0.0001**) were found at each time point.
Conclusion: We have successfully demonstrated high efficacy bacterial reduction using a visible light sterilization system. The VLS showed statistical significance against both gram positive and gram-negative species within the given treatment times. The β-lactam antibiotic-resistant E. coli showed very significant reduction levels which suggests that light therapy could a suitable option for sterilization in drug resistant species of bacteria. Our research illustrates the potential of using VLS in treating clinically relevant bacterial infections in vivo.
I. Lou1, A. Harrison1, A. Dammalapati1, R. Jaskula-Sztul1,2, H. Chen1,2 1University Of Wisconsin,Madison, WI, USA 2University Of Alabama,Birmingham, Alabama, USA
Introduction:
Medullary thyroid cancer (MTC) is a neuroendocrine tumor that when metastatic portends only a 40-50% overall 5-year survival. MTC commonly metastasizes to the liver, and when diffusely disseminated remains a therapeutic challenge as there are currently no curative options. The aim of this study is to determine the feasibility of an in vivo model of MTC liver metastasis.
Methods:
TT, a human MTC cell line, was genetically engineered to overexpress Notch3, a tumor suppressor in MTC, in the presence of doxycycline. Prior to injection, the cells were plated, and treated with doxycycline every 48 hours for 12 days to ensure adequacy of the inducible construct in vitro. 1×107 TT-Notch3 cells were injected intra-splenically into male nu/nu mice. The cells were allowed 2 minutes to enter circulation, and a splenectomy was performed. The mice were then recovered, given a standard chow diet, and the tumors were allowed to propagate. Physical examination of the mice at 4, 6, and 8 weeks revealed no external signs of intra-abdominal tumor burden such as ascites or palpable masses. Each mouse was imaged via computerized tomography (CT) scan with Fenestra VC contrast agent (MediLumine Inc, Montreal, Quebec) to evaluate for presence of tumors at 12 weeks. The mice were then sacrificed to determined correlation with CT findings and gross specimens. Lastly, the liver tumors were quantified with the use of Inveon Research Workplace (IRW, Siemens Healthcare).
Results:
Western blotting analysis for the Notch3 protein revealed maintenance of induction in the TT-Notch3 cells over a period of 12 days in vitro. There was 100% survival of these mice at 12 weeks. When imaged, 100% of mice demonstrated the presence of liver metastasis on CT scanning with Fenestra VC contrast. The imaging results correlated well in 80% of mice on necropsy with grossly visible tumors (Figure 1). The tumors on CT scan were then quantified by volume measurements with the use of IRW software.
Conclusion:
The intrasplenic injection model is a feasible method in which to form, image, and quantify liver metastasis for MTC. This in vivo model is an important step in further understanding metastatic MTC, and in the discovery and development of future therapeutics.
A. A. Mrazek1, M. Falzon2, J. Zhou2, M. R. Hellmich1, C. Chao1 1University Of Texas Medical Branch,Surgery,Galveston, TEXAS, USA 2University Of Texas Medical Branch,Pharmacology And Toxicology,Galveston, TEXAS, USA
Introduction: Chronic pancreatitis (CP) is progressive disease involving irreversible histologic damage and pancreatic insufficiency. CP is the result of repeated acute pancreatitis RAP, where damaged pancreatic tissue is replaced with scar. There is no cure for CP, and current treatment options are limited to supportive care and symptom palliation rather than targeting disease pathogenesis. Development of a novel therapeutic that reduces the severity of RAP would thereby prevent or delay progression to CP. We have found that the naturally-occurring flavanoid, apigenin, induced a protective phenotype in a preclinical mouse model of RAP, preserving pancreatic architecture and limiting stromal fibrosis. As a potential molecular target, we investigated the effect of apigenin on MAPK pathway activation during pancreatitis.
Methods: RAP was induced in 6-8 week-old C57BL/6 mice (Harlan Laboratories, Houston, TX) using serial cerulein (CR) injections: 50 μg/kg, 5 hourly intraperitoneal (IP) injections, 3 d/wk, for 4 wks (Bachem, Torrance, CA):. Apigenin treatment was initiated after one week of RAP-induced pancreatitis: 50 μg, oral gavage, once daily, 6 d/wk, for 3 wks (Sigma-Aldrich, St. Louis, MO). Control mice received the vehicles (saline IP, and 0.5% methylcellulose with 0.025% Tween20 by oral gavage) following the same schedule. Mouse pancreata were harvested, formalin-fixed, paraffin-embedded, and sectioned. Immunohistochemistry was performed using a heat-mediated antigen retrieval method, avidin/biotin blocking, phospho-extracellular signal-regulated kinase (pERK) antibody (Cell Signaling, Danvers, MA), VECTASTAIN Elite ABC kit and DAPI-containing mounting medium (Vector Lab, Burlingame, CA). Slides were counter-stained with hematoxylin (Thermo Fisher Scientific, Kalamazoo, MI). Ten non-overlapping 400x images of each pancreas and analyzed using the validated free-ware ImmunoRatio program, which calculated the percentage of positively DAB-stained nuclei. Nuclear translocation of pERK served as conjugate for MAPK pathway activation. Statistical analysis consisted of one-way ANOVA and post-hoc Tukey’s test.
Results: Basal pERK expression was predominately cytoplasmic within the vehicle and apigenin groups. CR-induced RAP resulted in significant MAPK pathway activation, i.e. pERK translocation to the nucleus (nuclear positivity). Daily 50 μg doses of apigenin, while continuing to induce RAP, resulted in a significant reduction of pERK nuclear translocation (p < 0.001) compared to pancreata treated with CR alone.
Conclusion: Apigenin inhibited MAPK pathway activation by limiting pERK nuclear translocation. The down-stream effector of the MAPK pathway, pERK, may serve as a transcription factor for the expression of genes regulating cell proliferation, differentiation, and apoptosis. Thus, down-regulation of the MAPK/ERK pathway is one of apigenin’s molecular mechanism by which pancreatic injury is reduced during RAP.
M. E. Stack1, C. Cockrell1, G. An1 1University Of Chicago,Department Of Surgery,Chicago, IL, USA
Introduction: Ulcerative colitis (UC) has an increased risk of colorectal cancer (CRC) due to the genomic instability caused by chronic inflammation, demonstrating a cumulative risk as high as 18% after 30 years of disease duration. Though sporadic CRC has a well described step-wise pattern of genetic and molecular alterations leading to malignancy, the dynamics of CRC arising in the setting of chronic inflammation is still not fully understood. In order to better understand the complex set of events leading to the development and progression of colitis-associated cancer, we will use a dynamic agent-based computational model (ABM) of colonic epithelium, termed the GI Cancer ABM (GICABM). The GICABM reproduces genetic events in colonic epithelium that lead to cancer, with the effect of inflammation manifested as increased rates of DNA damage. The GICABM is used to generate in silico patient populations, and is validated by matching epidemiological colon cancer rates seen in both sporadic and UC populations.
Methods: The GICABM is comprised of agents representing colonic epithelial cells and utilizes the DNA damage-repair functions from our prior oncogenesis ABMs. Rules for the GICABM were derived from existing literature concerning genetic factors associated with colon cancer. DNA damage was generated by a variable probability of mutation in a group of thirteen genes which have been implicated in the development of colon cancer: p53, telomerase, K-Ras, EGFR, TGF-beta, APC, Beta-catenin, PIK3CA, DCC, E-cadherin, SMAD4, BRAF, and C-src. The effect of chronic inflammation was modeled by increasing the rate of DNA damage. Sporadic colon cancer rates were retrieved from the SEER database, and a literature survey was used to generate a normalized risk progression for UC patients.
Results: The GICABM produced in silico populations (N = 1 million patients) over 60 years of simulated time that effectively reproduced the epidemiological data for both sporadic and UC CRC, with UC patients exhibiting a 2-5 fold increase in CRC risk when compared to sporadic CRC rates. Analysis of mutational patterns reproduced known patterns of mutational progression for sporadic CRC, and further suggested a conserved sequence for UC CRC involving impairment of DNA repair, followed by proliferation/immortality mutations.
Conclusion: The GICABM effectively reproduces existing data concerning sporadic CRC, and extrapolates the behavior of UC CRC. It allows visualization of the complex, lengthy processes involved in oncogenesis, and provides insight into mutational and evolutionary dynamics that lead to cancer under different conditions. This GICABM can potentially augment more traditional oncology research as both a hypothesis generating tool as well as a means for in silico hypothesis testing.
A. Chacon1, B. E. Phillips1, S. Kelleher1, M. Chacon1, D. Brunke-Reese1, D. Soybel1 1Penn State University College Of Medicine,Hershey, PA, USA
Introduction: Chemical and mechanical irritation of the peritoneum lead to acute inflammation, delaying recovery of intestinal motor coordination and increasing susceptibility to secondary infection. Recent studies in a variety of settings have provided evidence that targeted therapy with purified omega-3 fatty acids or synthetic pro-resolution lipids may attenuate acute inflammation and hasten the transition to healing. Administration of highly processed nutraceutical agents may, however, be attended with unanticipated toxicities or undesirable pharmacologic effects. In this study, we tested the hypothesis that resolution of peritoneal inflammation would be enhanced by pre-treatment with fish oil rich in docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), precursors to more potent lipid mediators of resolution.
Methods: C57/BL6 mice were begun on a daily oral gavage of 200uL Marinol® C-38, a fish oil blend containing 17% DHA and 22% EPA (FO) or saline as a control (C). At day 7, 3% thioglycollate media was injected into the peritoneum to create a chemical peritonitis, and exudates were collected by peritoneal lavage at 0hr (no injection), 48hr, and 96hr. Lipid inflammatory mediators in lavage fluid were assessed via ELISA (Leukotriene B4 {LTB4}, Resolvin D1 {RvD1}). Cells harvested via lavage were isolated for differential Wright-Giemsa staining, and cells collected at 96hr (95% macrophages) were assessed for macrophage differentiation markers via quantitative PCR. Additional cells were incubated in media alone for 16hr or with the addition of LPS (100ng/ml). Ex vivo cytokine secretion was assessed in culture media via ELISA (IL-6, IL-10, IL-1β, TNFα), and phagocytic capacity was assessed via uptake of fluorescent E. coli bioparticles.
Results: Fish Oil (FO) increased the ratio of RvD1/LTB4 in the peritoneal cavity at 0hr. No significant changes were observed in total counts of polymorphonuclear cells, macrophages or lymphocytes in the lavage fluid, and FO did not significantly alter mRNA markers of macrophage phenotype. FO interestingly increased ex vivo TNFα secretion after stimulation with LPS in cells harvested at 96hr. No effect of FO was seen on LPS-stimulated particle phagocytosis.
Conclusion: Our findings provide evidence that nutraceutically relevant doses of FO supplements enhance production of pro-resolution lipid mediators such as RvD1 relative to that of pro-inflammation mediators such as LTB4 following induction of chemical peritonitis, without altering counts or transition states of local innate immune cell populations. These observations stand in contrast to more powerful and global effects of purified compounds, suggesting that the latter may be more effective in enhancing resolution, but also in inviting unwanted pharmacologic sequelae. Further studies will be required to determine whether FO-induced alterations in the RvD1/LTB4 ratio can hasten resolution without compromising other homeostatic functions.
01.20 Ultrasonic, Bipolar, and Integrated Energy Devices: Comparing Heat Spread in Collateral Tissue
M. G. White1, M. K. Applewhite1, B. C. James1, H. Safiuddin1, L. Abdulrasool1, H. Jamil1, E. L. Kaplan1, P. Angelos1, R. H. Grogan1 1University Of Chicago,Endocrine Surgery Research Group, Department Of Surgery,Chicago, IL, USA
Introduction: The use of energy devices in surgical procedures has become common after the introduction of bipolar and ultrasonic energy devices in the late 1990s. Recently, integrated laparoscopic devices, which simultaneously combine bipolar and ultrasonic energies, has resulted in increased interest in this technology for open surgery. This study was performed to quantitatively compare heat transfer and collateral tissue damage that may be associated with these devices.
Methods: Three serial firings for each setting of the bipolar (Ligasure Small Jaw), ultrasonic (Harmonic Focus), and integrated (Thunderbeat Open Fine Jaw) devices were applied in vivo to various porcine tissue types including: liver, muscle, and thyroid. Thermocouplers were inserted into surrounding tissue at 1 mm intervals to measure temperatures over time as the devices were fired. Temperature was reported in degrees Celsius. Recurrent laryngeal nerve (RLN) monitoring was performed to determine the practical implications of the heat generation in tissue. This was done with firings of each device at successively decreasing distances from the RLN until signal was lost. Statistical analysis included t-tests and ANOVA was performed using STATA.
Results: The amount of heat generated in the tissue between the lowest and highest settings was similar for each device tested (p=0.11-0.81). However, when comparing heat generated across these devices for the peak temperature reached at 1 mm, the peak temperature recorded in liver tissue was observed with HF (103.4 ± 44.1), in muscle tissue – with OFJ (57.9 ± 16.9); and in thyroid – with LS (54.8 ± 25.1). RLN signal was lost after firing 1mm away from the RLN for all devices while nerve monitor signal was not affected by firing at ≥2 mm.
Conclusion: Comparable quantitative data on heat dispersion and collateral tissue damage by surgical energy devices is one of the important factors that surgeons use to make informed decisions regarding the devices they choose for surgery. Our findings conclude that heat transfer as a function of depth was similar among all three devices with the exception of HF resulting in significantly higher temperatures than OFJ and LS in the liver. Liver tissue showed the most consistent results between experiments therefore we recommend that liver tissue be used for any future experiments on heat transfer to tissue. Although collateral heat transfer is an important consideration, tissue cutting speed and dissection capability are also key factors when choosing a surgical device for clinical applications. Further studies are required to evaluate all relevant performance attributes in a controlled clinical environment.
N. M. Kunda1, J. Qin1, G. Qiao1, B. Prabhakar2, A. V. Maker1,2 1University Of Illinois At Chicago,Division Of Surgical Oncology, Department Of Surgery, College Of Medicine,Chicago, IL, USA 2University Of Illinois At Chicago,Department Of Microbiology & Immunology, College Of Medicine,Chicago, IL, USA
Introduction:
We have previously demonstrated that human and murine colon cancer cells undergo near complete cell death in vitro and in vivo upon direct exposure to PV-10 (10% rose bengal disodium), a synthetic dye currently in clinical trails for intralesional therapy of in-transit melanoma. Occasional bystander responses have raised the possibility that PV-10 induced cell death can generate an anti-tumor immune response. The mechanism of PV-10 cell death in not known, and it is critical to determine, if rose bengal disodium is to be used as an immunotherapeutic strategy for solid tumors.
Methods:
Murine colon cancer CT-26 cells were treated with PV-10 concentrations of 0 – 300 μM for 24 hours. For cell cycle studies, cells were fixed with 80% ethanol and DNA staining was performed using DAPI. DNA content was measured with FACS and cell cycle distribution was analyzed. GRP78 and LC3 were analyzed using Western blot. Protein levels of GRP78 were measured to evaluate ER (Endoplasmic Reticulum) integrity, and the conversion of LC3-I to LC3-II was determined as an indicator of stress-induced autophagy.
Results:
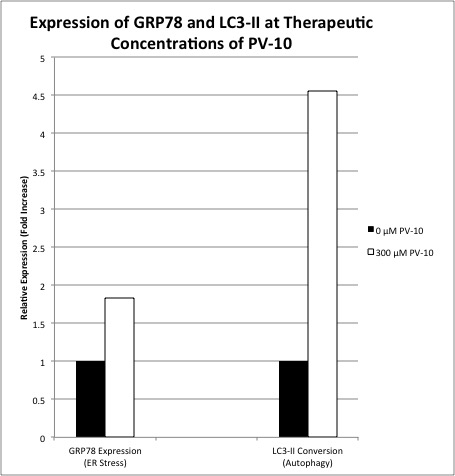
At clinical concentrations of PV-10, the fraction of cells in the G2-M phase increased from 21%-43% (p=0.05), and the fraction of cells in the G1 phase decreased correspondingly from 58% to 30% (p=0.06), consistent with cell cycle growth arrest. The fraction of cells in the sub-G1 phase increased over 4-fold from 3% to 14% (p=0.04), consistent with DNA degradation and apoptosis. GRP78 expression increased almost 2-fold from 1 to 1.83 upon PV-10 exposure, indicative of ER stress. Similarly, LC3-II expression increased almost 5-fold from 1 to 4.53 with PV-10 exposure, consistent with autophagy-induced cell death (Figure 1).
Conclusion:
Treatment of colon cancer cells with PV-10 induced cell cycle arrest, apoptosis, autophagy, and significant ER stress; consistent with immunogenic apoptosis. In order for cytotoxic agents to act as potential immunotherapeutic strategies in the treatment of solid tumors, immunogenic cell death targeting the endoplasmic reticulum (ER), leading to ER stress may be critical. Therefore, based on these results, further evaluation of PV-10 as a potential agent to stimulate immunologic cell death in solid tumors is warranted.
S. A. Morrison1, S. Hamarneh1, H. Sturgeon1, D. Hu1, H. Huo1, W. Zhang1, K. Economopoulos1, S. Gul1, F. Adiliaghdam1, J. Ramirez Decrescenzo1, R. Hodin1 1Massachusetts General Hospital,Surgery,Boston, MA, USA
Introduction: Inflammatory Bowel Disease (IBD) remains a chronic disease of major clinical significance secondary to its increasing prevalence and morbidity worldwide. While the exact mechanistic pathogenesis of IBD remains elusive, its origins are known to be both complex and multi-factorial; involving variants in gene expression, exposure to environmental agents, and a dysregulated immune response. At the heart of suspected disease expression lies the tenet that aberrant gut-derived immune factors play a central role in pathogenesis. This work is based on the hypothesis that potential decreased expression of the brush border enzyme, Intestinal Alkaline Phosphastase, known for its anti-inflammatory properties, may contribute to disease development in these populations.
Methods: Colonic biopsy samples were obtained from 17 patients with Inflammatory Bowel Disease, as well as from 10 healthy controls. In IBD patients, specimens were obtained both from inflamed and non-inflamed areas. Inflammatory cytokine levels and IAP mRNA was determined by quantitative reverse transcription-polymerase chain reaction.
Results:When compared with tissue from healthy controls, IAP mRNA expression was decreased in both inflamed and non-inflamed samples taken from patients with Ulcerative Colitis (UC), with the most significant decrease seen in inflamed tissue( Healthy Controls: 1.15, UC non-inflamed 0.85 (p=0.07), UC inflamed 0.19 (p=0.01)) . In a smaller sample size, tissue samples from Crohn’s patients did not reveal a significant difference in IAP expression when compared to healthy controls (Healthy Controls: 1.15, Crohn’s disease inflamed and non-inflamed: 1.16, 1.02). Inflammatory cytokines TNF-alpha and IL-8 were higher in IBD patients, specifically in inflamed tissue, with expression in Ulcerative Colitis patients being particularly high, correlating to lower levels of IAP, a gut derived anti-inflammatory enzyme (IL-8: HC 0.82, CD non-inflamed and inflamed 8.4 and 276 (p=0.2 and 0.002), UC non-inflamed and inflamed 22.5 and 3441 (p=0.06 and 0.007) TNF-a: HC 0.91, CD non-inflamed and inflamed 1.76 and 6.24 (p=0.05 and 0.006), UC non-inflamed and inflamed 9.17 and 47.3 (p=0.002 and p=0.01)).
Conclusion: Decreased levels of IAP expression, correlating with increased cytokine levels in the inflamed mucosa of patients with Ulcerative Colitis may indicate a role for the enzyme in disease pathogenesis and could lay groundwork for use of this agent as a therapeutic modality in future clinical studies.
G. Karagkounis1,2, J. Zhao3, X. Li3, M. F. Kalady1,2 1Cleveland Clinic,Stem Cell Biology And Regenerative Medicine,Cleveland, OH, USA 2Cleveland Clinic,Colorectal Surgery,Cleveland, OH, USA 3Cleveland Clinic,Immunology,Cleveland, OH, USA
Introduction:
Our group has previously shown that interleukin-17 (IL-17) supports colorectal cancer stem cells, enhancing their viability and growth. Studying these effects in normal colonic mucosa is challenging due to the limited availability of in vitro models. Recently developed complex culture systems allowing the maintenance and expansion of multicellular structures derived from colonic mucosa, also known as colon organoids or epithelioids, have allowed new insight in colon tumorigenesis and response to inflammation. The goal of this study was to use colon organoids to investigate whether IL-17 promotes stemness in normal colonic mucosa.
Methods:
Freshly isolated mucosa without evidence of malignancy was collected from human colon according to an established IRB-approved protocol. Samples from different areas of the colon were cultured independently and expanded in media containing R-Spondin, Noggin, EGF and Wnt3a. Each cultured sample was split in two grossly equal fractions that were subsequently treated with either IL-17 (100ng/ml) or vehicle control. After 24 hours, the organoids were harvested and mRNA was isolated. Real-time quantitative PCR (RT-qPCR) was used to measure mRNA expression levels for interleukin-6 (IL-6), a known target of IL-17 used as positive control, as well as stem-cell markers ALDH1, and CD44. Paired t-test was used to compare expression levels between stimulated and control organoids.
Results:
Organoid cultures were established successfully and expanded for 10 days before experiments were performed. IL-17 stimulation resulted in significant upregulation of IL-6 expression compared to control (fold change 18.1, p=0.003). In addition, IL-17 induced CD44 (fold change 1.5, p=0.04), and ALDH1 (fold change 1.7, p=0.03).
Conclusion:
In this proof-of-concept study, inflammatory stimulation in the form of IL-17 induced stem-cell associated genes in normal colonic mucosa. Colon organoids enable in vitro studies of tumorigenesis and allow the assessment of mucosal response to various stimuli. These findings highlight the potential of this model and provide an opportunity for further studies to elucidate the role of IL-17 in the development of cancer.
A. H. Choi1, Q. Xing1, J. Yan1, W. Wen1, E. S. Han2, J. H. Yim1 1City Of Hope National Medical Center,Division Of Surgical Oncology,Duarte, CA, USA 2City Of Hope National Medical Center,Division Of Gynecologic Oncology,Duarte, CA, USA
Introduction: The mammalian target of rapamycin (mTOR) signaling pathway controls fundamental cellular processes, including cell proliferation and metabolism. mTOR phosphorylates ribosomal protein S6 kinase 1 (S6K1), activating ribosomal protein S6 (S6), which is involved in cellular proliferation and glucose metabolism. Metformin has been shown to disrupt the mTOR pathway and is currently the subject of an ongoing phase III randomized trial as adjuvant treatment in breast cancer (NCT01101438). The flavone baicalein, which is present in the herb thyme and in asian herbal remedies, appears to be non-toxic in animals and humans. We have previously demonstrated that baicalein effectively inhibits cancer cell proliferation by inhibiting mTOR signaling. The objective of this study was to compare the effect of baicalein with metformin in triple-negative breast cancer.
Methods: MDA-MB-468, a triple-negative breast cancer cell line, was treated with baicalein (0-80 uM) and metformin (0-10,000 uM) for 24-72 hours. Total protein lysates were obtained for immunoblot analysis of the mTOR pathway mediators S6K1 and S6, as well as the mTOR inhibitor DNA Damage-Inducible Transcript 4 (DDIT4). Cytotoxicity after 72 hours of treatment was evaluated by MTT assay.
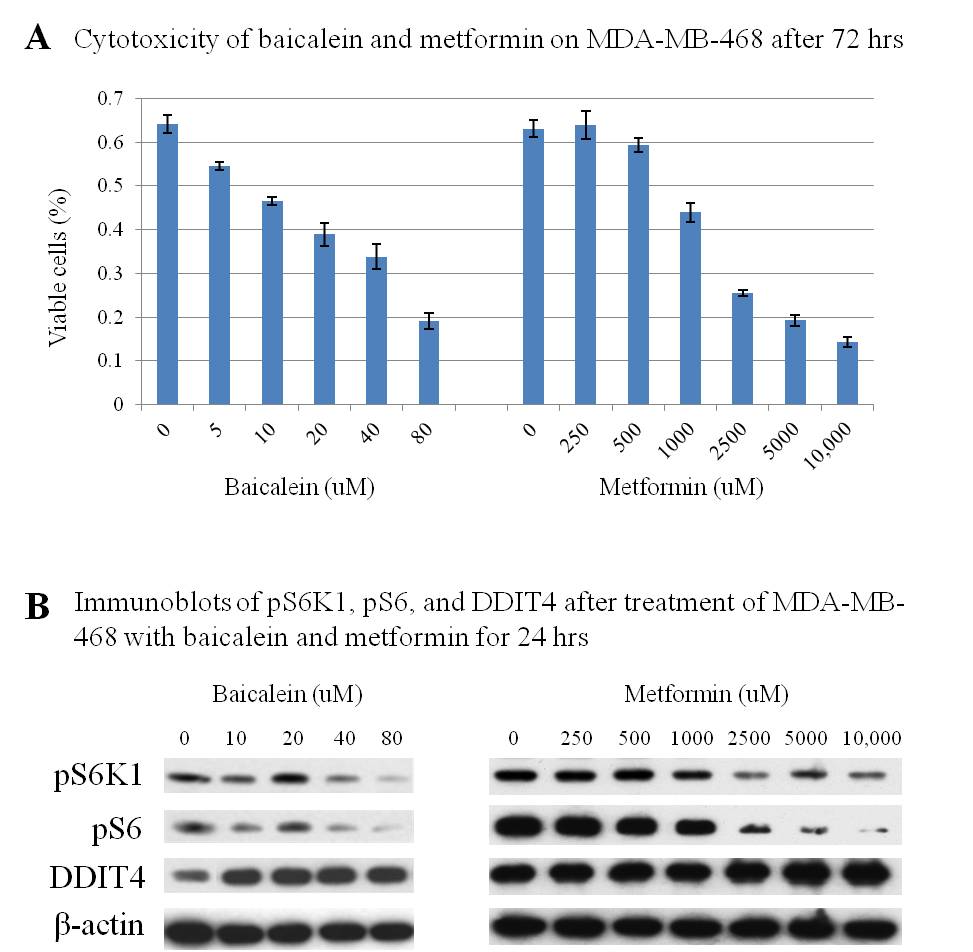
Results: Baicalein demonstrated dose-dependent growth inhibition of MDA-MB-468 in micromolar concentrations. By comparison, metformin required over a 60-fold higher concentration to achieve similar cytotoxicity (Figure 1A). Dose-dependent inhibition of phospho-S6K1 (pS6K1) and phospho-S6 (pS6) were observed following treatment with both baicalein and metformin. However, baicalein-induced phosphorylation blockade was more effective at a far lower concentration. The activity of DDIT4, an mTOR inhibitor, increased with higher concentrations of baicalein (Figure 1B).
Conclusion: Baicalein effectively inhibits growth of triple-negative breast cancer in vitro. Dose-dependent pS6K1 and S6 inhibition, as well as increased DDIT4 levels, were observed at micromolar concentrations. Baicalein, a flavone that appears to be non-toxic in humans, may represent a novel treatment for triple-negative breast cancer.
H. Jin1,2, M. Roy1, A. Dammalapati1, A. Harrispn1, A. Ma1, R. Jaskula-Sztul1,2, H. Chen1,2 1University Of Alabama,Surgery,Birmingham, AL, USA 2University Of Alabama,Surgery,Birmingham, Alabama, USA 3University Of Wisconsin,Surgery,Madison, WI, USA
Introduction: ~~It is known that Notch signaling is minimally active in neuroendocrine (NE) cancer cells and the induction of Notch isoforms alter the malignant neuroendocrine phenotype. Although the induc¬tion of Notch1 by Histone Deacetylase Inhibitors (HDACi) appeared to be the result of increased Notch1 expression at the transcriptional level, the effects of HDACi on the Notch1 promoter regulation have not been determined thus far. The aim of our study is to investigate the molecular mechanism of HDACi activation on the Notch1 pathway.
Methods: ~~We functionally characterized the Notch1 promoter using deletion mapping. The mapping started with the truncated genomic DNA fragment fused with a luciferase reporter, transfected into BON cell, a carcinoid cell line, screened for luciferase activity. Protein-DNA binding was then performed by electrophoretic mobility shift assay (EMSA).
Results:~~Two HDACi, Valproid Acid and Tailandepsin-A, were shown to induce luciferase activity controlled by a small distal region of Notch1 promoter, from -80 to +1 of the start codon ATG. Further, we identified a functional DNA motif that is responsible for HDACi induction located at -75 to -55 of the Notch1 promoter region. Thus, an in vitro assay, EMSA revealed the transcription factor-DNA complex firmed in the flanking sequence.
Conclusion:~~We have identified the DNA motif located in the Notch1 promoter region that is responsive to HDACi. This understanding of how HDACi act on the Notch1 promoter may lead to the development of future novel therapies for neuroendocrine cancers.
H. Aoki1,2, M. Aoki1,2, P. Mukhopadhyay1,2, C. C. Barnett3, S. Spiegel1,2, K. Takabe1,2 1Virginia Commonwealth University,Division Of Surgical Oncology, Department Of Surgery,Richmond, VA, USA 2Virginia Commonwealth University,Department Of Biochemistry & Molecular Biology,Richmond, VA, USA 3University Of Colorado Denver,Department Of Surgery,Aurora, CO, USA
Introduction: In the U.S., pancreatic cancer is the 4th leading cause of cancer death in both gender. The 5-year relative survival rate is approximaly 6% for all stages. Up to 60% of the patients are found to have distant metastatic disease at the time of diagnosis, at which median survival is around 6 months. The most common sites for distant metastases are the liver (80%), peritoneum (60%), lung and pleura (50-70%), and adrenal glands (25%). Prognosis of patients with peritoneal carcinomatosis (PC), dissemination of cancer cells throughout the abdominal cavity, are particularly poor with median survival of only 6 weeks. This poor overall 5-year survival rate has not significantly changed over the past 5 decades, which reflects the fact that there is no effective treatment available for this condition. Sphingosine-1-phosphate (S1P), a bioactive lipid mediator produced by sphingosine kinase 1 (SphK1) and sphingosine kinase 2 (SphK2), plays critical roles in many aspects of cancer progression, such as cell proliferation, migration, angiogenesis and lymphangiogenesis. We have recently published that S1P link inflammation and cancer in colitis-associated cancer progression (Cancer Cell 2013). Given the fact that inflammation is known to be essential for establishment and progression of PC, where cancer cells need to adhere to the peritoneum and form a nodule, we hypothesized that S1P levels regulated by SphK1 and SphK2 in the host animal may have different mechanism in promoting progression of pancreatic cancer PC depending upon its levels.
Methods: Murine pancreatic adenocarcinoma panc02-luc cells were intraperitoneally injected into SphK1 wild type (WT) or knockout (KO), or SphK2 WT or KO mice to generate PC model. Tumor burden was quantified using bioluminescence imaging. Survival was assessed by Kaplan-Meier analysis. PC nodules were harvested 14 days after injection and analyzed. The proliferation was assessed by Ki-67 staining and apoptosis was evaluated by TUNEL assay. We also compared mRNA expression by RT-PCR.
Results: Circulating S1P levels were lower in SphK1 KO mice and higher in SphK2 KO mice compared with respective littermate WTs probably due to compensatory elevation of SphK1. Panc02-luc cells developed significantly less tumor burden, less inflammatory cell infiltration, and less cancer cell proliferation, but with no difference in apoptosis in PC of SphK1 KO mice. These results suggest that host S1P promotes PC progression by stimulation of proliferation of cancer cells. Interestingly, SphK2 KO mice developed less tumor burden, longer survival with elevated CD4 and CD8 lymphocyte infiltrates in PC.
Conclusion: Our results implicate an intriguing possibility that S1P levels in the host may have different mechanisms in promoting progression of pancreatic cancer PC depending upon its levels.
